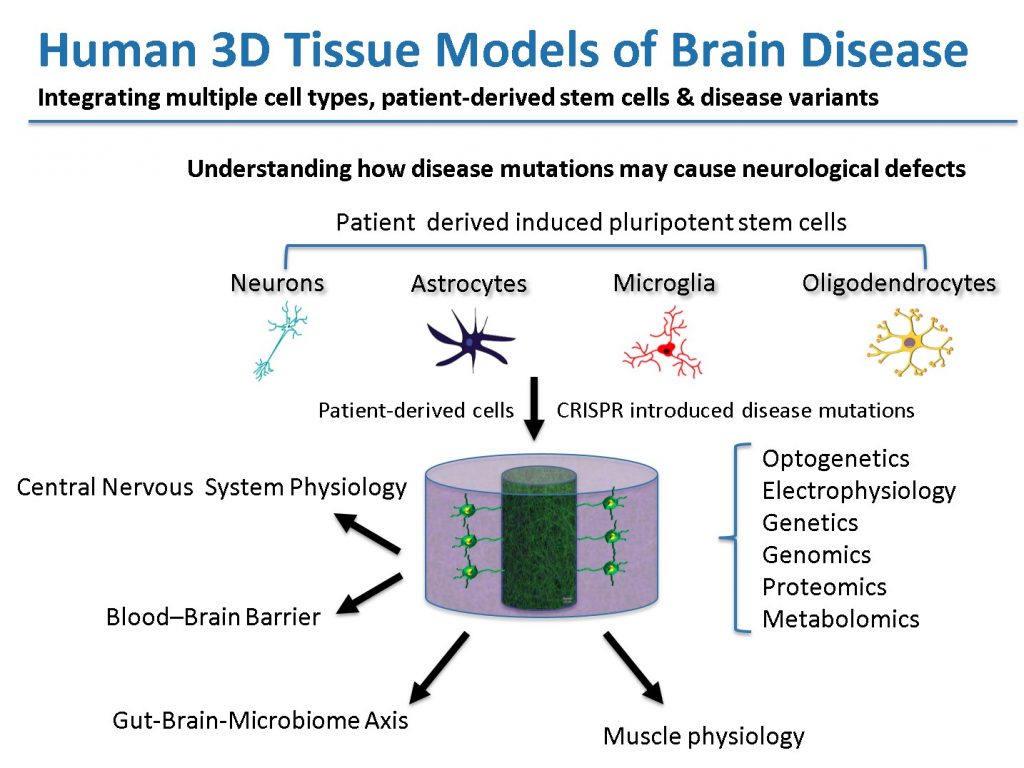
Our approach to study human brain disease is based on engineering disease relevant 3-D brain tissue models based on induced pluripotent stem cells (iPSCs) from patients with brain diseases and iPSCs that carry specific disease mutations introduced by CRISPR. We make use of biocompatible scaffolds, such as silk and extracellular matrix hydrogels, for the targeted arrangement of cells using 3-dimensional tissue engineering principles. These technologies enable us to reconstruct the architecture, function and heterocellular composition of diseased neural circuits (e.g., cortical–striatal, cortical–dopaminergic networks) and composite systems (blood-brain barrier and the brain–intestine–immune axis). We use iPSC technology to derive specific neuronal cell types (cortical, striatal, dopaminergic, and enteric nervous cells) and support cells (astrocytes, microglia, and oligodendrocytes) and characterize the outputs of our models using a variety of techniques (optogenetics, electrophysiology, genetics, and “-omics” approaches).
Establishing 3D models of brain diseases provides more accurate models for in vitro study. It is possible to model and characterize the basic cellular network features of functional disease states, such as impaired learning in Alzheimer’s Disease, and screen therapeutic approaches. In addition, environmental exposures have been implicated in several neurologic diseases and disorders, including Parkinson’s Disease (PD) and Autism Spectrum Disorder (ASD). Future studies can quantify candidate toxicants and characterize metabolic responses and pathway alterations in 3D brain tissue models. This coupled approach can be applied to a range of other exposure scenarios, including drug delivery (e.g., nanoparticles) and mixed agents, to assess efficacy, synergy, and potential negative impacts.
Projects include:
Alzheimer’s Disease Tissue Engineered Brain Models