Paper 1 – Clinical efficacy and immune regulation with peanut oral immunotherapy13
Introduction: With the prevalence of food allergies on the rise, the search for improved methods to help control allergic reactions has been a focus of the scientific community. Currently, avoidance diets are an unsatisfactory, since there are still many risks for accidental contact with dangerous allergens.
Oral immunotherapy (OIT) is thought to be a substantial method for creating gradual desensitization to food allergens, with desensitization being classified as a gradual increase in the threshold of tolerance for ingest food protein. Within this paper, the results of a clinical trial of desensitization to peanut protein Ara2 are discussed.
Children ages 1 through 16 years old from Duke University Medical center and Arkansas Children’s Hospital underwent OIT in order to quantify the clinical efficacy of this form of therapy, as well as to test the hypothesis that increasing desensitization to the peanut allergen would shift subject’s immunological profiles towards a Th1-dominated profile (downregulation of Th2 cells). The oral immunotherapy procedure was broken up into three main phases: initial day escalation, the build-up phase, and the maintenance phase.
Initial Day Escalation: Doses of peanut protein Ara H2 were given to the study subjects in intervals of 0.1 mg, with the dose doubling every 30 minutes until 50 mg was reached. The highest tolerated dose for each individual subject was made their starting dose for the build-up phase. Peanut protein was mixed into a food (decided by the subject) and taken in 2-3 bites.
Build-up Phase: Subjects ingested their determined minimum tolerated dose with other safe foods at home daily. Doses were increased by 25 mg every 2 weeks until a maximum of 300 mg was reached. For patients whose initial tolerated doses were less than 50 mg, their doses were doubled every two weeks until 50 mg was tolerated; following this, their doses also increased by 25 mg each two weeks. Dosage increases were performed at local clinics; dosing was delayed if there was evidence of external health problems (viral infections, illness, etc.).
Maintenance Phase: Subjects continued to ingest 300 mg of peanut protein until an oral food challenge was performed after 12 months. Subjects were reevaluated every 4 months while in the maintenance phase, for a total of 36 months.
Oral Food Challenge: Subjects underwent an OFC to peanut protein after a time period ranging from 4 – 22 months in the maintenance phase. This range was determined by the results of preliminary basophil and skin test data. The oral food challenge involved 4 doses of peanut protein – 300 mg, 600 mg 1200 mg, 1800 mg – given every 30 minutes until a maximum of 3.9 g of peanut protein was reached.
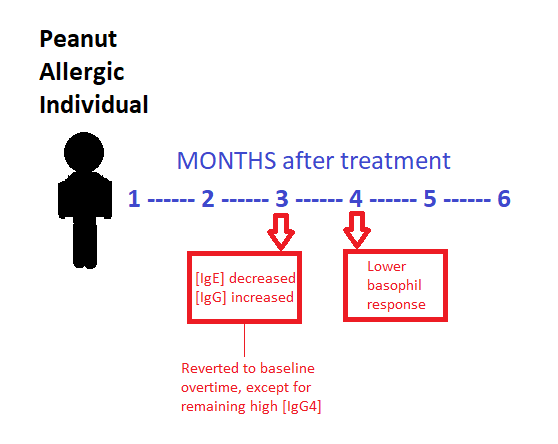
Figure 22 – Summary of impact of oral immunotherapy on a peanut-allergic patient
Results: Out of the 29 subjects who participated in the oral food challenge to peanut protein, 27/29 (93%) were able to tolerate the maximum dose of peanut protein (3.9 g) with only mild symptoms. Titrated skin prick tests, performed every four months following the maintenance phase, showed a significant decrease in allergic response throughout the study beginning at 6 months. Similarly, basophil activity due to peanut allergen showed a significant decrease after 4 months of treatment and continued to decrease throughout the study, indicating a lowered allergic reaction to increasing concentrations of peanut protein.
Measurements of immunoglobulin concentrations revealed that peanut-specific IgE declined over an 18 month period, while IgG and IgG4 increased. IgG4 antibodies are involved in blocking antigens from binding with IgE, preventing IgE mediated allergic reactions from occurring. The decrease in IgE combined with the simultaneous increase in IgG4 indicates a successful inhibition of IgE-mediated allergy symptoms.
While it was hypothesized that oral immunotherapy would result in the downregulation of allergen-specific Th2 cells and upregulation of Th1 cells, the increase in inflammatory cytokines and chemokines did not reflect the transition to a Th1 immunological profile (as expected). It was proposed that this change from a Th2 dominated profile to a proinflammatory profile may have been the result of oral vs. subcutaneous (applied under the skin) exposure to peanut protein. Additional studies would need to be performed to investigate this difference.
Overall, this clinical trial of oral immunotherapy showed successful desensitization to peanut protein in peanut-allergic children who completed at least 8 months of OIT. Oral immunotherapy could thus be a method of creating a larger margin of safety for those with food allergies. Additional studies are currently underway to determine if OIT can be used to create long-term tolerance to certain food allergens, and whether the downregulation of genes in apoptotic pathways shown in T cell microarrays could lead to further insights into the mechanism of oral immunotherapy.
So as IgG4 remained higher in concentration, does this mean that the individuals as a result also had less severe reactions or had less sensitivity to peanuts? Was the lower basophil response reverted as well? What did this treatment really do for the patients as a result?
I would include what the actual treatment looks like i.e. how often it is ingested and what the relevant chemicals involved are. I also think this page could benefit from an explanation of why IgG4 concentrations remained high and what that did for patients given that it lasted for many months after treatment.