First, how do peanut allergies work in general?
The molecular basis of peanuts’ allergenicity is a relevant topic, personally and societally. Peanuts contain various proteins, but the ones shown to be relevant to allergic reactions are Ara h 1, Ara h 2, and Ara h 34. Research into the immunological activity of these proteins shows that Ara h 2 is one of the more harmful proteins and is relatively resilient in the face of gastrointestinal digestion – thus we’ve chosen Ara h 2 as our protein to explore.
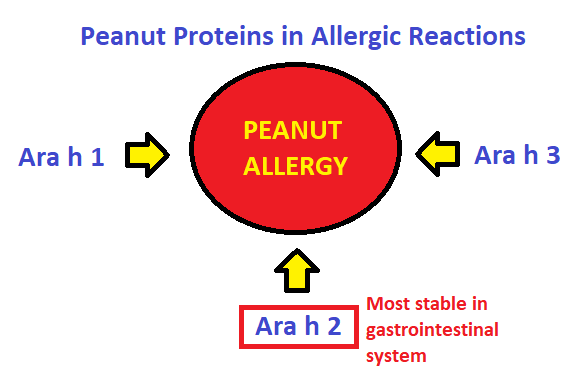
Figure 2 – selection of peanut protein for study
Allergic reactions in general are considered a type of hypersensitivity reaction, specifically a Type I response. In these responses, the body acts as if the allergen is a harmful pathogen5. This is due to the production of IgE antibodies that respond to peanuts, an otherwise innocuous antigen.
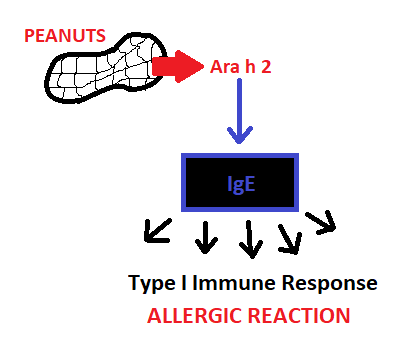
Figure 3 – summary of immune response generation
Various therapies are currently being used or developed to mitigate food-related Type I hypersensitivity. Those that apply specifically to the body’s response to peanuts and Ara h 2 protein include those that inhibit IgE production or the effector pathways initiated when Ara h 2 protein and IgE interact, those that desensitize an individual to the allergen’s effect, and those that compete for IgE’s active site5. The most common treatment is peanut oral immunotherapy, which aims to reduce, if not eliminate, the patient’s adverse reaction to peanuts.

Figure 4 – general therapeutic methods for combating allergic responses to peanuts
Second, how do peanut proteins, in particular Ara h 2, produce hypersensitivity?
The Ara h 2 protein’s structure is shown in Figure 5 (in red), along with the maltose-binding protein (MBP) used by researchers as a carrier protein to aid in crystal structure formation (in green). Ara h 2 is made up of fives helices, held in place by four disulfide bonds between cysteine residues (in yellow). 6
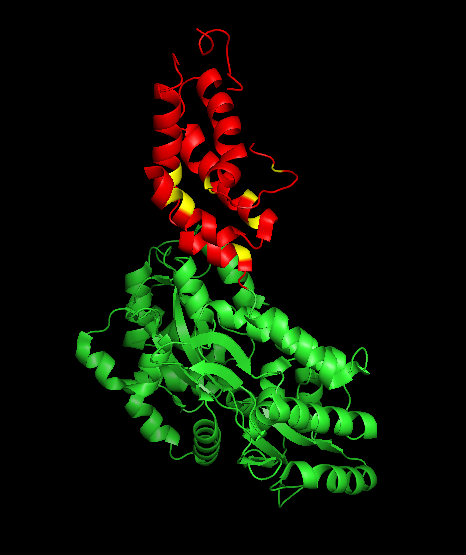
Figure 5 – crystal structure of Ara h 2, showing carrier protein in green and Ara h 2 helices in red, with cysteine residues participating in disulfide bonds in yellow7.
Ara h 2 is a member of the prolamin protein family, a group of plant seed proteins with high concentrations of proline and glutamine residues. This makes these proteins insoluble in water6, 8 . The primary structure of Ara h 2 is shown in the figure below.
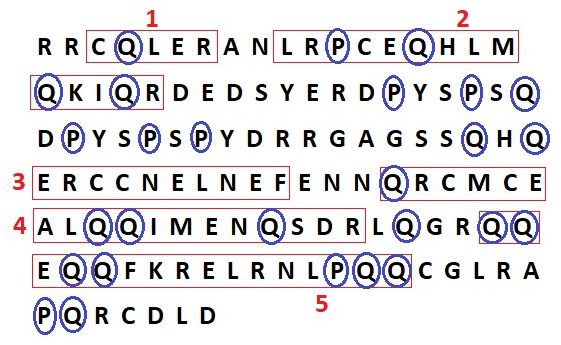
Figure 6 – shows the primary sequence of Ara h 2; each alpha helix of the molecule is outlined in red and numbered, while all proline and glutamine residues are circled in blue to demonstrate the high concentration characteristic of prolamins
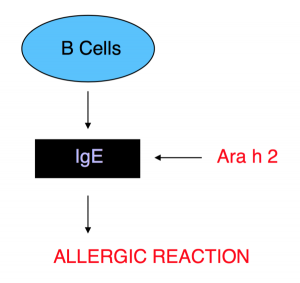
In an allergic reaction, the Ara h 2 protein interacts with an immunoglobulin E (IgE) antibody. The exact biochemical pathway by which this interaction occurs can be found here. IgE is produced in humans and other mammals by B-cells, a specialized type of white blood cell (lymphocyte).
IgE is distinguished from other immunoglobulins by the amino acid content of its constant region (Fc)9. All immunoglobulins are made up of paired light and heavy polypeptide chains, forming a Y-shaped structure, as shown in Figure 8d. The hinge of the Y is formed as the light chains associate with the heavy chains – each arm of the Y is a portion of light chain associated with the amino-terminal half of the heavy chain, while the carboxy-terminal portions of the heavy chain associate together in the base of the Y. The immunoglobulin’s Fc can be found within the heavy polypeptide chain, mostly in that base region. The light chains contains a constant domain and a variable domain, both classified either as kappa or lamda. These structural features are shown in Figures 8a and 8c, in which the paired heavy chains are shown in yellow and blue, with the light chain in pink. The structure of IgE’s Fc specifically is shown in 8b, with the A and B domains colored yellow and blue, respectively. Because all immunoglobulins have comparable structures, the Fc chain of IgE may be contextualized within the entire structure of immunoglobulin G (IgG), shown in 8a5.
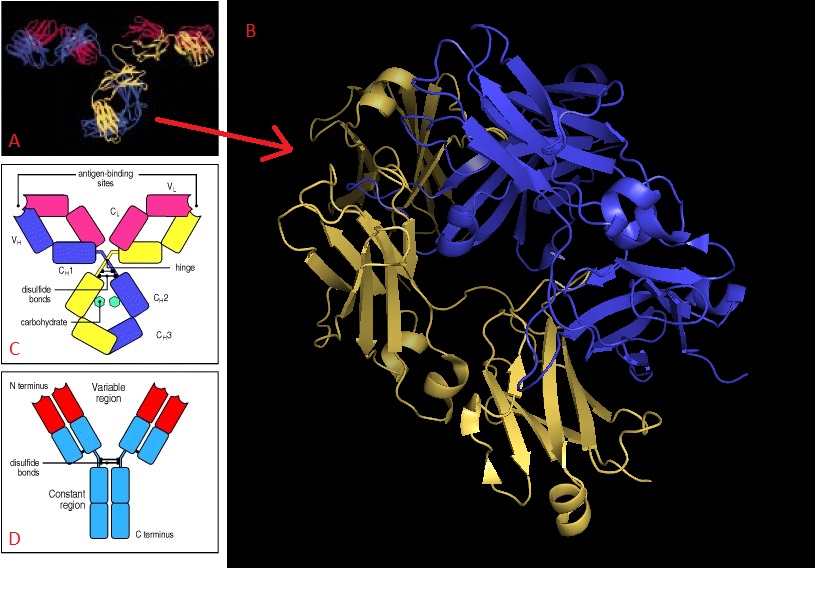
Figure 8 – structure of immunoglobulins; part (a) shows entire structure of IgG, with heavy and light chains visible, while (b) shows a closer view of the heavy chain constant region for IgE. Parts (c) and (d) offer schematic views of IgG’s overall structure. In (c), the antigen binding sites are indicated, as are the light (CL/VL) and heavy (CH, VH) chains. In (d), the N- and C-termini of the protein’s heavy chain are noted. 5, 10
The Fc of IgE is the portion which binds it to a mast cell’s plasma membrane during an allergic response, at a high-affinity receptor known as FcƐRI. Ara h 2 interacts with IgE at various epitopes – the regions of an antigen at which the antibody binds11. The details of the Ara h 2 epitopes and how they were identified can be found here.
Ara h 2 cross links with IgE, prompting the antibody to bind to the mast cell receptor, FcƐRI. That binding initiates a cascading immunological reaction5. This – the way in which a peanut protein causes an allergic response – is summarized in Figure 9, below.
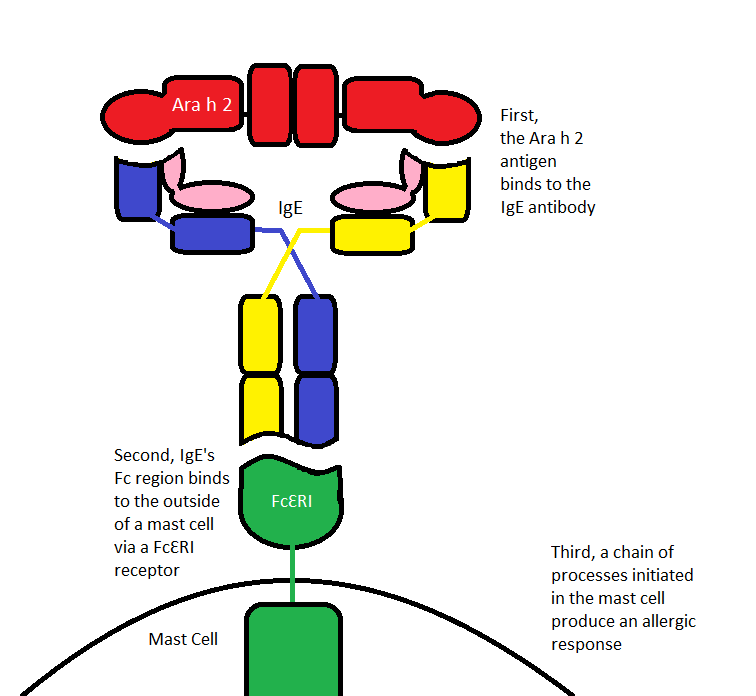
Figure 9 – a summary of the interactions leading to a peanut-allergic response prompted by Ara h 2
IgE was discovered as the result of an observed allergic response after a blood transfusion, which indicated that a factor in the blood prompted allergic response. One hypothesis as to its purpose is that IgE originally evolved as a defense against parasites. In fact, parasites do elicit IgE production by immune cells. Another hypothesis posits that IgE plays a role in early recognition of foreign particles. Thus, IgE may be produced in any individual but is only produced when a response is required. This response is concentration dependent – only if a sufficient number of cross-linked IgE antibodies bind to immune cells will the response cascade begin12.
Third, how do therapies for peanut allergy biochemically mitigate the immune response triggered by Ara h 2?
While there are no permanent solutions to peanut allergies, there are a few therapies currently in use that decrease the allergic response to peanuts overall. One therapy that frequently appears in current research, testing, and treatment is peanut oral immunotherapy. While peanut oral immunotherapy is not yet a common therapy, it is used to research possible treatments for peanut allergies. Peanut oral immunotherapy aims to desensitize patients to the allergen. What the various oral immunotherapy tests have in common is for patients to ingest peanut protein daily (alongside a safe food), increasing the dose slowly over a certain period of time13. The dosages of peanut protein and the time periods over which the patients are examined vary with each test. Patients’ allergic responses are recorded and blood is sometimes drawn for more testing.
Through these tests, it has been found that, over time, peanut-specific IgE decreases and IgG4 increases 14. This increase in IgG4 is found especially in three immunodominant epitopes in Ara h 2 (epitope 3, 6, and 7), which are found in most patients with symptomatic peanut allergies15. Figure 10, below, shows the way in which IgE decreases and IgG4 increases with desensitization to the allergen.
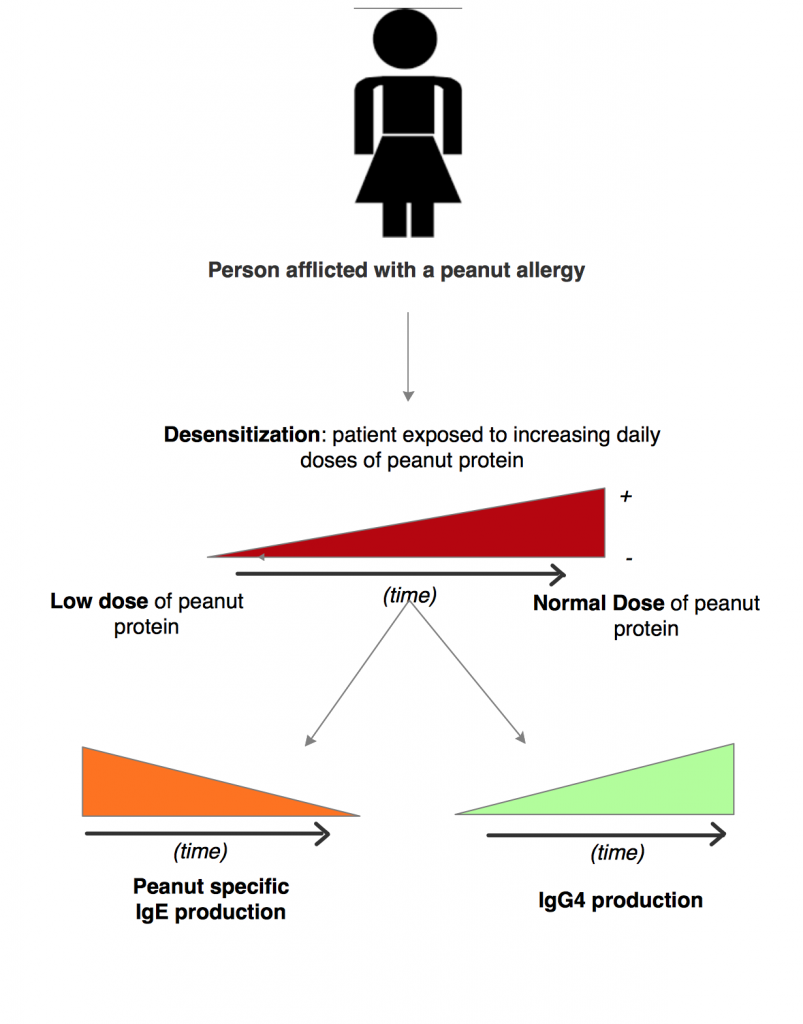
Figure 10 – A summary of oral immunotherapy effects on patients allergic to peanut protein.
Among the various treatments available for peanut allergy, the strategy of desensitization via peanut oral immunotherapy has proven to be promising. It does not truly cure a peanut allergy, but it is able to mitigate the symptoms, in part by decreasing the amount of peanut-specific IgE in the body. With less IgE present to be cross-linked by Ara h 2, the signalling cascade that provokes an allergic response in the body has a harder time getting started, leading to fewer and less severe reactions to the allergen.
This portion is a great segue into the more specific chemical pathway involved. However, a couple sentences did feel a bit drawn out in the peanut oral immunotherapy section. The figures help a lot with following the logic as does the examination of the primary structure of Ara h 2 with respect to its higher level structure and in the immune/allergic response.
I think this page does a great job at outlining the methods/approach you took to answering your overall question. I’m not sure how essential the Pymol diagrams are, but they do help provide context for the tertiary structure and overall size of the immunoglobulin. The last figure is also very useful and clear, perhaps I would include a short explanation following it in order to help emphasize the conclusion/takeaway in terms of therapeutic treatment.