Inherited Risk of Diabetes in Mice
Yanchang Wei, et al. “Paternally Induced Transgenerational Inheritance of Susceptibility to Diabetes in Mammals.” Proceedings of the National Academy of Sciences, vol. 111, no. 5, 2014, pp. 1873–1878. [22]
EXPERIMENTAL DESIGN
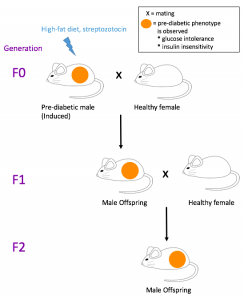
Pre-diabetes in male mice (F0) was induced by a high-fat diet and low dose of streptozotocin. Clinically, streptozotocin is a drug used to treat carcinoma in pancreatic beta cells, and while it destroys cancer cells, it also inhibits the ability to secrete insulin and causes high blood glucose levels. [23] The scientists observed prediabetic symptoms, such as reduced glucose tolerance and insulin sensitivity, in these mice. The pre-diabetic and control male mice were mated with healthy female mice, and those offspring were then mated again to breed another generation. The offspring (F1) of the pre-diabetic mice and the succeeding generation (F2) exhibited the same metabolic phenotype as F0. A closer investigation revealed that methylation patterns in the pancreatic islets of both offspring generations were similar to those of the F0 sperm. This finding suggests that with the exposure as non-genetically induced prediabetes, the F1 and F2 generations may have inherited epigenetic marks of methylation from the F0 mice.
Figure 1. Summary of the experiment. Adapted from ref. [4]
METHYLATION LEVELS OF PANCREATIC ISLETS IN F1 OFFSPRING
Overall, paternal prediabetes appeared to change the expression of 402 genes in the pancreatic cells of the offspring, including both upregulation and downregulation. Many of these genes affected insulin signaling and glucose metabolism. Genes with significant results were related to insulin signaling. Two coded for phosphatidylinositol (PI) 3-kinase subunits, Pik3ca and Pik3r1. The third gene, Ptpn1, coded for a tyrosine phosphatase that inhibits insulin signaling. The degrees of expression were measured using mRNA levels. The methylation patterns of the genomes of the pancreatic islets of the F1 and F2 generations were determined using methylated DNA immunoprecipitation sequencing (MeDIP-Seq). Compared to the control, analysis in the F1 offspring of pre-diabetic mice revealed increased methylation at the intragenic regions of Pik3r1 and Pik3ca, which led to downregulation. On the other hand, hypomethylation was observed in the intragenic region of Ptpn1, which led to upregulation. Furthermore, similar epigenetic marks were also observed in the offspring (F2) of the pre-diabetic F1 mice. Overall, the decreased expression levels of Pik3r1 and Pik3ca and increased levels of Ptpn1 impaired insulin signaling in the offspring of pre-diabetic mice.
METHYLOMES OF F0 SPERM AND F1 PANCREATIC ISLETS
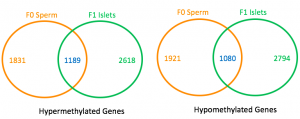
The sperm methylomes of the control and pre-diabetic F0 mice were determined using bisulfite sequencing, which is another method to identify methylated DNA regions. As seen with the pancreatic islets of the offspring, increased methylation in Pik3ca and Pik3r1 was observed in the sperm of pre-diabetic mice, although methylation of Ptpn1 was not decreased. Furthermore, the overall methylation patterns of the F0 sperm and F1 pancreatic islets were similar — 39 percent of the intragenic regions of the hypermethylated genes in the F0 sperm were also hypermethylated in the F1 cells, while 36 percent of those of hypomethylated genes in the F0 sperm were also hypomethylated in the F1 cells. Figure 3 shows these overlaps. With these similarities, the authors conclude that the F0 sperm methylome strongly predicts patterns in the offspring.
Figure 3. Overlaps of differentially methylated regions between F0 sperm and F1 pancreatic islets. Adapted with permission from publisher from reference [22] . © 2014 PNAS. For each Venn-diagram, the orange number and green number indicate the numbers of genes that are differentially methylated in the F0 sperm and F1 islets, respectively. The blue number shows the number of genes that are differentially methylated in both cells.
CLOSER LOOK: METHYLOMES OF F0 SPERM AND F1 BLASTOCYSTS
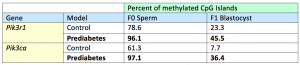
The final question was whether the methylated marks in the F1 pancreatic islets were due to de novo methylated by DNMT 3 or were simply inherited from the sperm. To answer this, the methylomes of F0 sperm and F1 blastocysts at the early stage of embryogenesis were determined and compared using bisulfite sequencing (Bis-Seq), and compared to controls, the pre-diabetic sample exhibited higher methylation levels at Pik3r1 and Pik3ca. This result may be evidence that the offspring inherited the methylated alleles from the sperm.
Table 1. Results of Bis-Seq of Pik3r1 and Pik3ca in F0 sperm and F1 blastocysts. Adapted with permission from publisher from reference [22] . © 2014 PNAS.The percentages indicate the proportion of CpG islands throughout the gene that are methylated. For both genes, a high methylation level in the sperm of the pre-diabetic F0 father predicted high methylation in the F1 blastocyst. This data suggests that the methylated marks on the F1 sperm remained throughout gametogenesis, fertilization, and embryogenesis.
CONCLUSION: THE STABILITY OF THE METHYLATION MARKS
During the early embryogenesis of mammals, genome-wide demethylation of the parental DNA is observed. [24] In a separate study, immunostaining of mouse zygotes revealed that while most genomic elements are demethylated, some regions retain their methyl groups. [24] This retainment may be the mechanism for epigenetic inheritance. [24] This research on pre-diabetic mice and their offspring indicates that the hypermethylated regions of the genes Pik3r1 and Pik3ca might have evaded postfertilization, genome-wide demethylation and were inherited from the F0 mice.
Leave a Reply