A study of Vietnam veterans found that many of the methylated CpG’s linked to PTSD symptoms in the F0 germ line were found in the TGF-β signaling pathway (2). Transforming growth factor beta (TGF-β), encoded by the TGFβ1 gene, is a secreted protein that performs many cellular functions including cell growth, cell proliferation and cell differentiation. To accomplish these functions, TGF-β1 has a complex signaling pathway vital to the immune system and neuronal health.
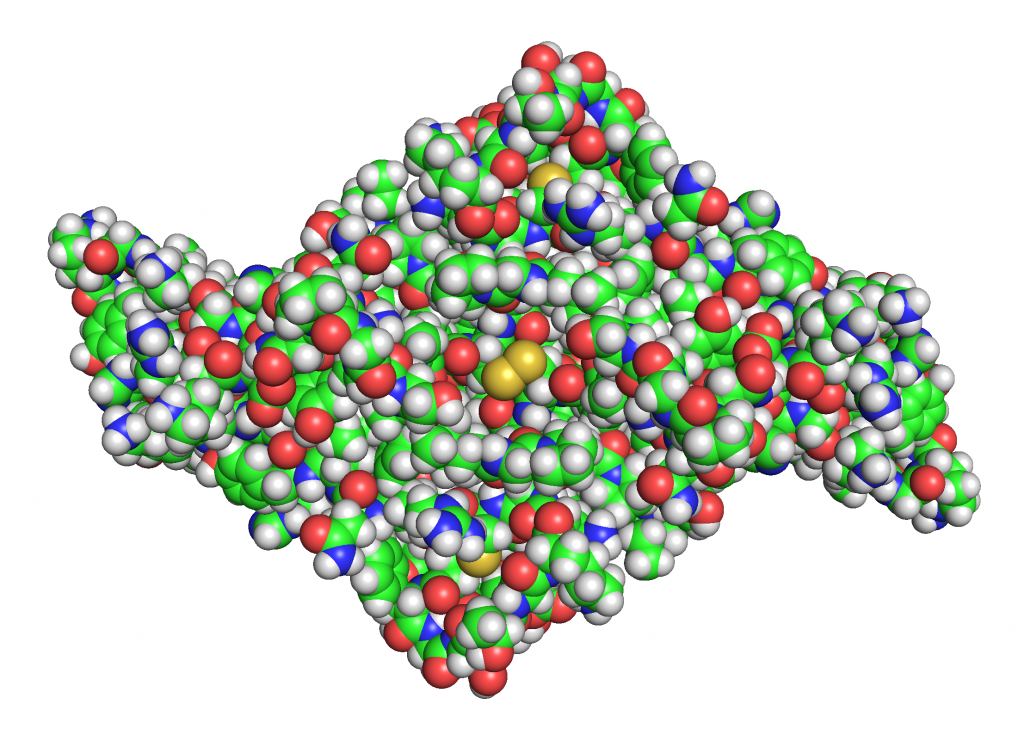
TGF-β is a homodimer composed of two identical polypeptide chains. The protein is primarily stabilized by hydrophobic interactions with additional support from 9 disulfide bonds (pairs of cysteines) spread throughout the center of the molecule. Each monomer consists of several β-sheets and 3 conserved disulfide bonds that form a cysteine knot which further stabilizes the protein structure (15). The two monomers are connected to each other via a central disulfide bond.
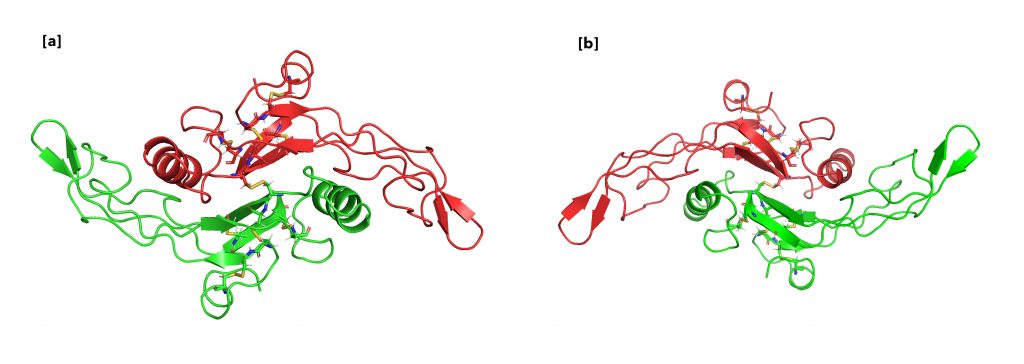
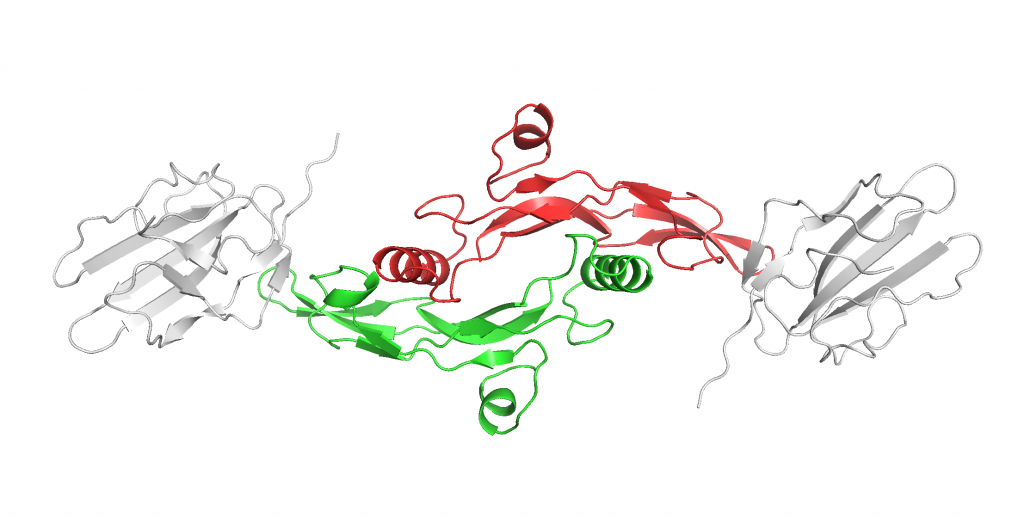
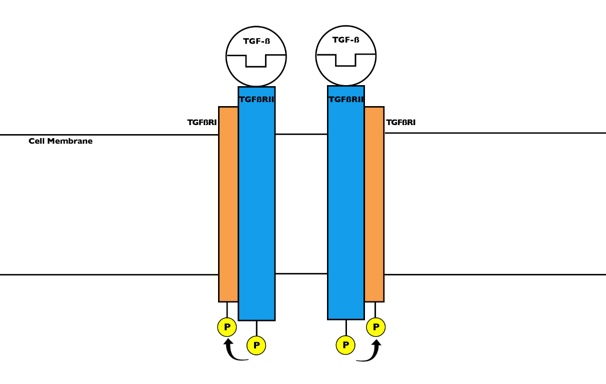
As shown in Figure 13, TGF-β primarily binds to 2 TGF-β type II receptors (TβRII), encoded by the TβRII gene, to initiate its wide array of functions in the cell by acting as a protein kinase. Figure 14 depicts that TβRII further recruits and phosphorylates TGF-β type I receptor (TβRI). The activated complex then is able to initiate the signaling pathway that results in all the cellular functions.
The study of Vietnam veterans found methylation in the genes of the TGF-β signaling pathway resulting in decreased levels of transcription. This consequently would also lead to decreased levels of TGF-β signaling. In order to confirm the effects of decreased TGF-β signaling, another paper mimicked the effects of which by simply deleting exon 2 of the TβRII gene, which codes for TβRII.
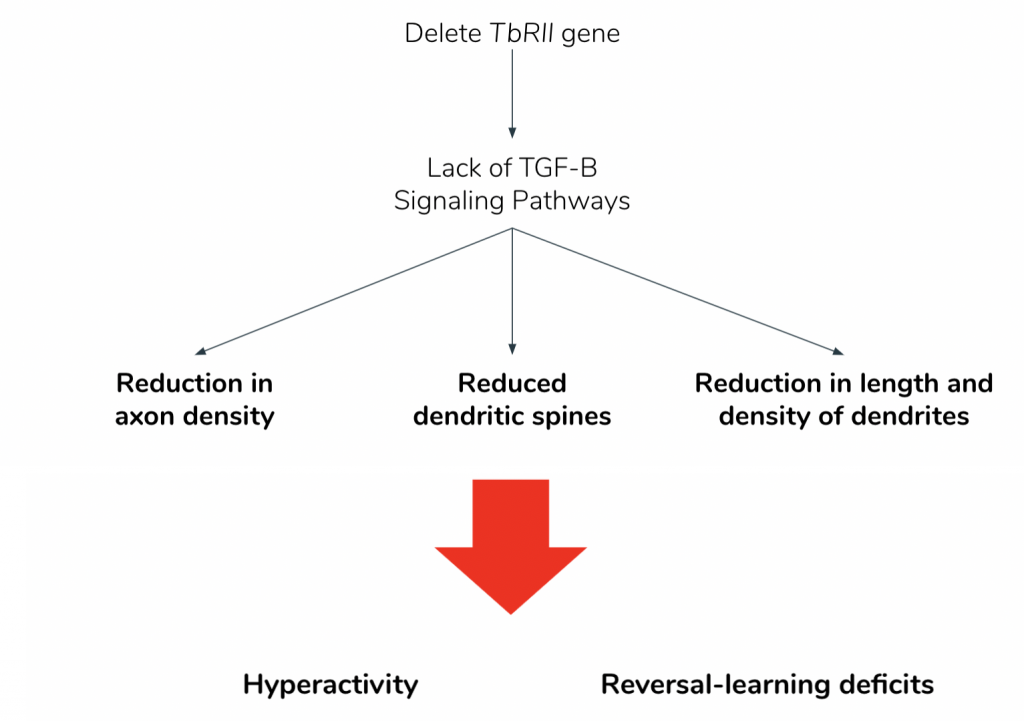
As showed in Figure 15, this deletion led to 3 main physiological effects. The dopaminergic (DA) neurons showed reduced axonal growth and reduced number of dendritic spines (6). Additionally, the mutants lacking the TβRII gene presented with reduced dendritic density and length (6). Loss of the TβRII gene similarly reduces the excitatory inputs and increases the inhibitory inputs in the DA neurons, thereby lowering the excitatory-inhibitory (E-I) ratio (6). During field tests, mice with lowered E-I ratio presented with hyperactivity, and a deficit for reversal learning, defined as the inability to update already-learned reward association with new information (6). These behavioral inflexibilities were consistent with the symptoms of neuropsychiatric disorders such as schizophrenia, ADHD, and PTSD (6).

This is really good in terms of figures and information. I think adding an overall bridging sentence to the beginning relating this to the PTSD patients could help focus the page, and then also adding whether this is in the peripheral/germline cells and/or if this effect is seen through to the next generation or if it is mainly in the F0 generation could be a good way to tie it to the rest of the project.
Thank you! We have added a sentence to bridge this page to the rest of the website. We also clarified the confusion in the paragraph as well. The effects of TGF signaling are seen in the F0 generation.
I really like how organized this page is. A small thing I noticed was that you mentioned CPG in this page instead of CpG islands, which was slightly confusing for me at the start. I really like the figures that you added because having the structure of the enzymes and how it binds to the protein really helps bring the page together. I agree with Claire that it would be good to note if this is the peripheral or the germline cells, especially whether or not this can be passed onto the next generation or if this is just the effect on the generation.
I was also slightly confused by Figure 14, are both of the molecules in the figure the same thing or is this another figure of the protein enzyme complex in Figure 13?
They are the same protein. I have updated the caption to clarify the confusion. Thank you!
In Figure 14., the yellow bonds are difficult to see. It would be helpful to make them more distinct (i.e. circle them, make them brighter, make them bigger, etc.). I think this would also be a good opportunity to add a “zoom-in” effect and add a small window to the figure showing the bonds from a closer angle. Otherwise, very easy to follow!
Thank you for your suggestion!
This page is clearly very well research, and the complexity is presented well. One thing that I got a little lost on while reading this page, however, was how it related to the presence of PTSD, or how it manifests itself in the offspring of veterans. Besides just saying at the end that it’s consistent with symptoms, how does it actually cause such symptoms?
The mechanisms of this action and further effects are still unknown since research into this field is still relatively new. I added a small portion to hopefully make the connection with PTSD more apparent.