Experiments
Determining the relationship between TGF-B and F1R1 in terms of cardiac cell proliferation & differentiation
The main goal of this experiment was to determine the relationship between TGF-B and F1R1 on a sample of ex-vivo neonatal rat pup cardiomyocytes. This was done by performing a cell culture with a control group of cardiomyocytes, a group treated with only F1R1, a group treated with only TGF-B, and a group treated with both F1R1 and TGF-B. These cells were cultured for 48 hours and experimental conditions were ran for another 48 hours. For more specific details on labwork methods, please refer to our 1st semester technical report.
These cells were stained with DAPI to detect the total number of cells in each sample, and then tagged with aSMA antibodies (to detect differentiation) and ki67 antibodies (to detect proliferation). Then, these cells were imaged using a Keyence microscope.

DAPI 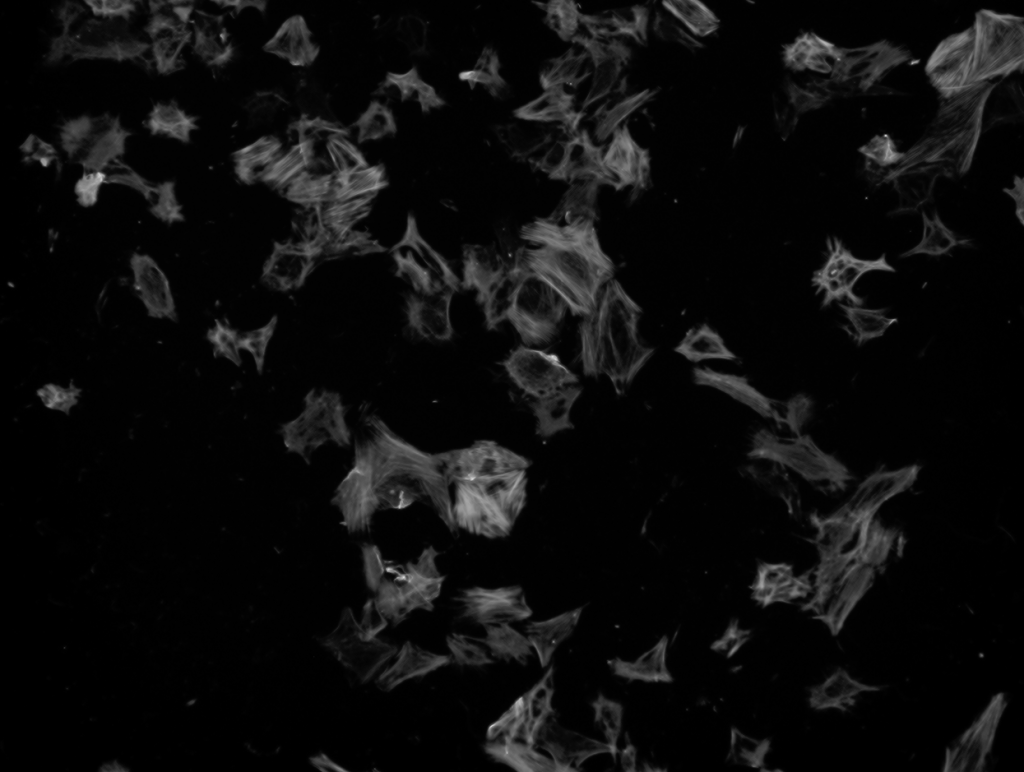
aSMA 
ki67 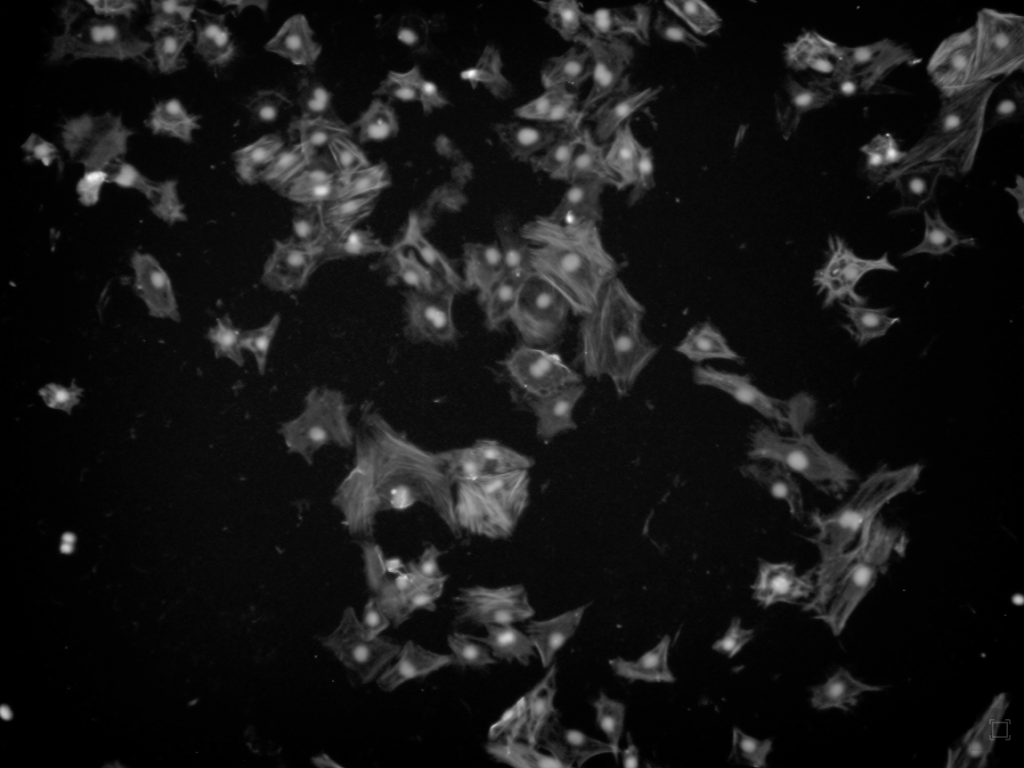
Overlay
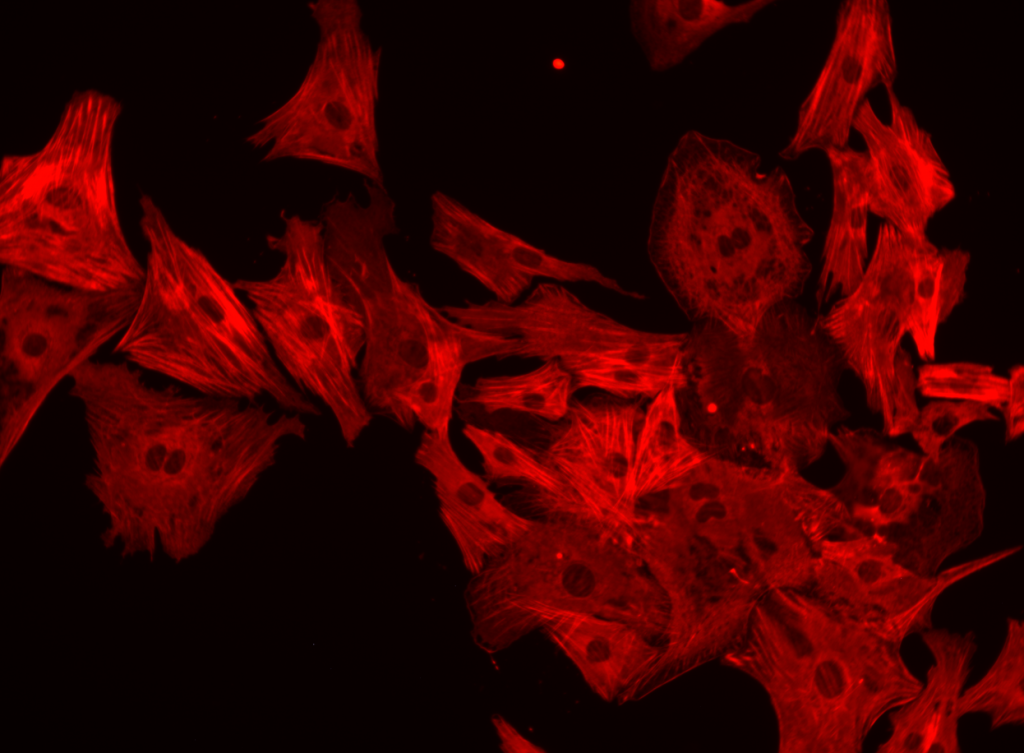
aSMA colored stain 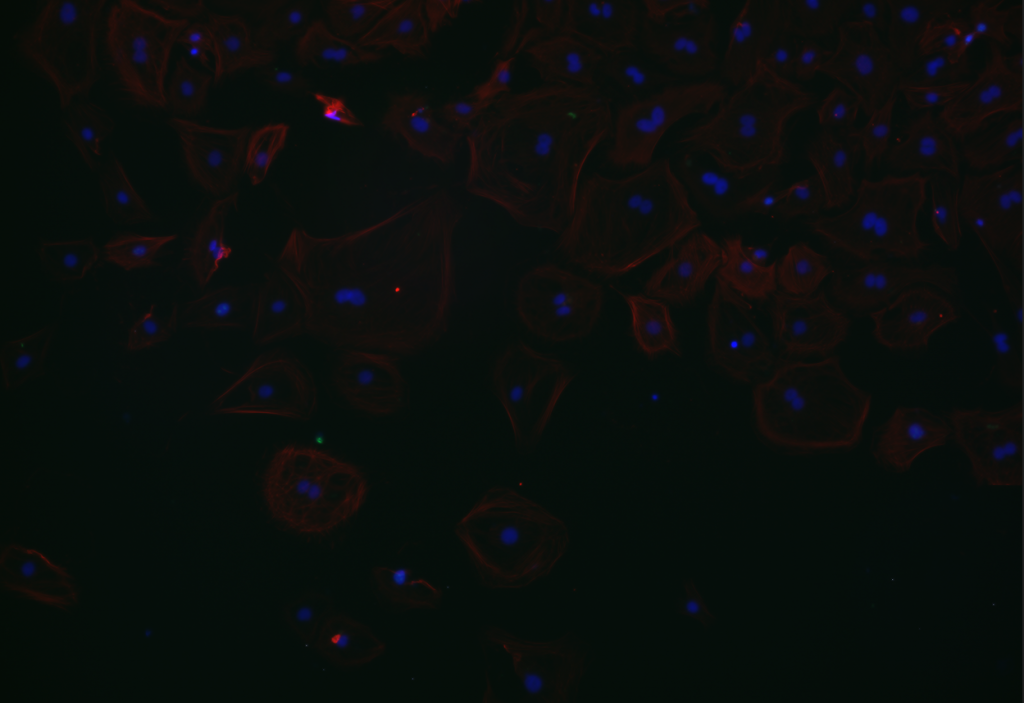
DAPI & aSMA colored stain
The darkfield images were then analyzed with CellProfiler to get accurate counts of total, proliferating, and differentiating cells in each of our 384 images.

DAPI 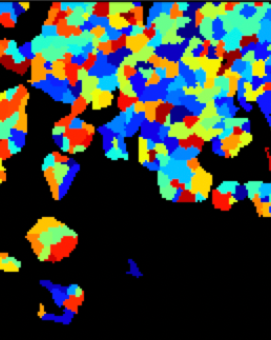
aSMA 
ki67 (identifying everything as an object EXCEPT the cells…) 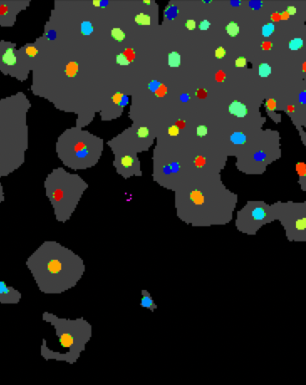
aSMA/DAPI overlay
Finally, the counts from CellProfiler allowed us to create a bar graph of differentiating cells (based on aSMA + cells / total DAPI-counted cells). We are currently working with some issues with detecting proliferation via the ki67 stain, but we will update this page with proliferation data once we obtain it.
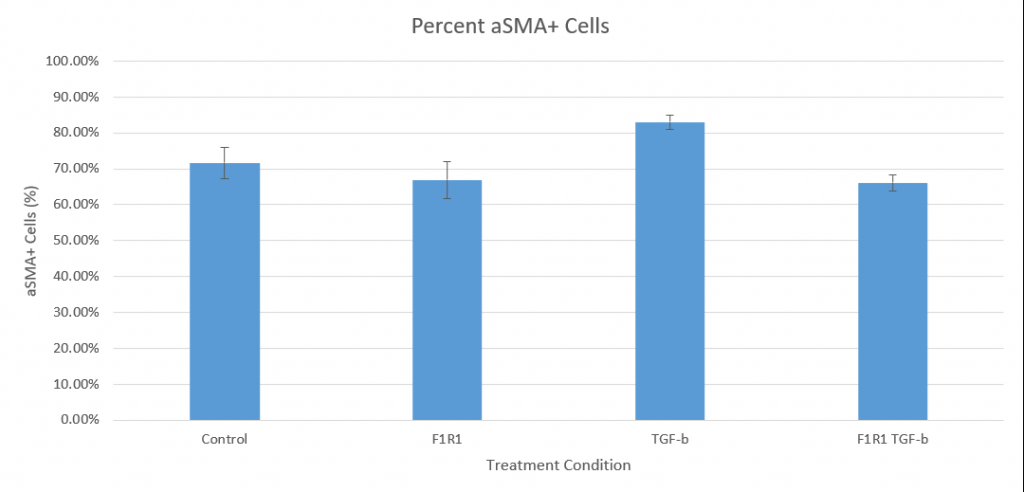
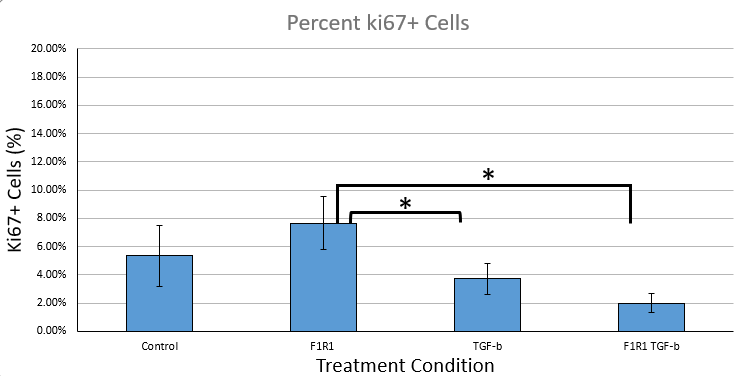
The TGF-B treatment group exhibited a greater average percentage of aSMA positivity than the control by 11.35%, confirming that the cardiac fibroblasts respond to TGF-B activation and differentiate. Furthermore, the F1R1/TGF-B condition had a lower average percentage of proliferating cells when compared to the TGF-B-only group by 16.9%. In this, the experimental evidence suggests that F1R1 can impede TGF-B activation of cardiac fibroblasts.
However, an unexpected result is the basal level of differentiation seen in the control. With no experimental stimulation, 71.7% of fibroblasts were positive for aSMA on average. A potential contributor to this could be the stress caused by the serum starvation stage performed during the culturing process.
Repeated Experiment w/ Serum-Containing Media
The only new element in this iteration of the experiment will be that the fibroblasts will be completely cultured in serum rather than including a serum starvation step. With this, we hope to reduce the amount of control differentiation so that the relationship between TGF-b and F1R1 can be more clearly seen in our experimental groups.
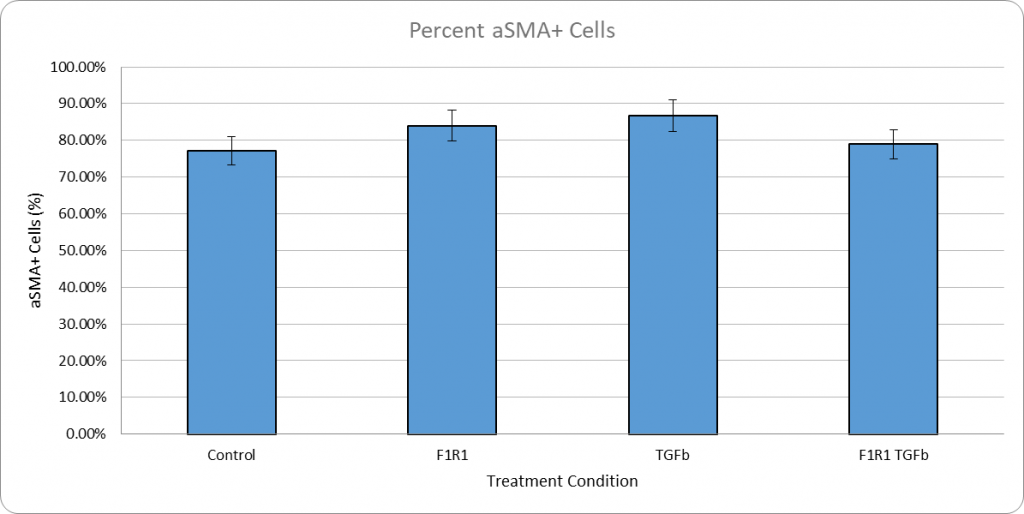
We were also able to get proliferation data with the ki67 stain in this experiment:
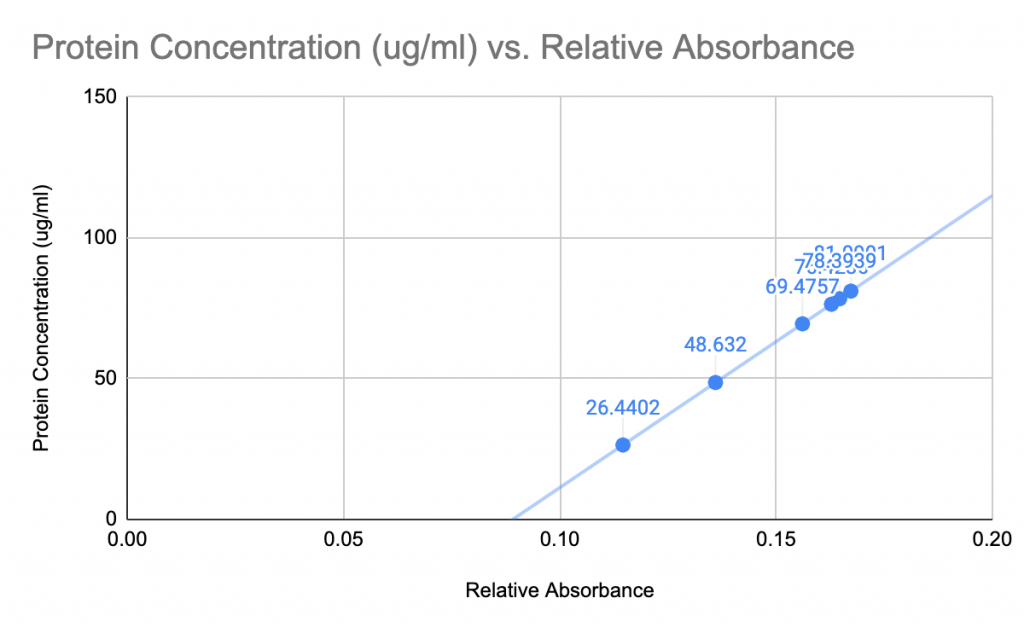
Cell Lysis & BCA Assay for Western Blot
Dose-Dependency of F1R1
*Note: This page is meant to give a brief summary of our experiments. For more details on methods, results, analysis, and implications, please refer to our technical reports and presentations under the “assignments” tab.