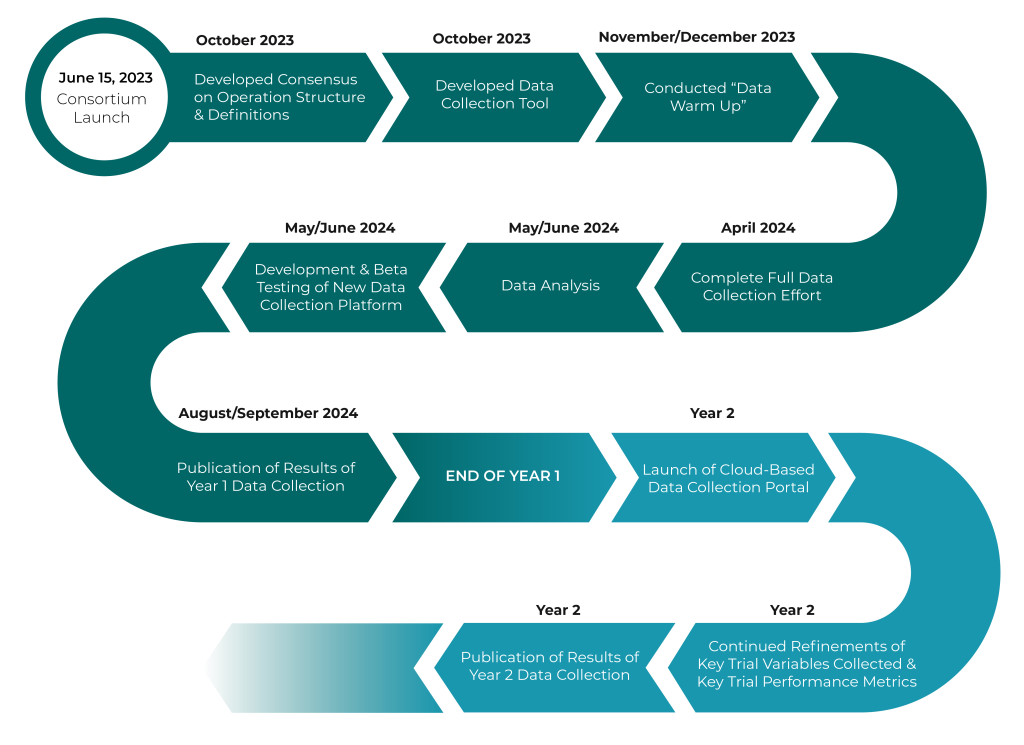
Launched in the summer of 2023, the Tufts Center for the Study of Drug Development (Tufts CSDD) established a pre-competitive consortium of pharmaceutical, biotechnology companies, contract research organizations, and niche service providers (called PACT – Partnership for Advancing Clinical Trials) to gather and analyze hard evidence on the use and impact of virtual and remote solutions supporting clinical trial planning and execution.
Tufts CSDD, based within the Tufts University School of Medicine, manages all aspects of PACT’s operations, acts as a coordinating center to compile and analyze data, facilitate and oversee member participation and consensus-building, and will help direct communication and dissemination of the study findings.
What are Decentralized Clinical Trials (DCT)? DCTs are clinical trials that use technology, processes, and/or services that carry the potential to reduce or eliminate the need for participants to physically visit a traditional research site. Examples of DCT activities include wearable devices, remote visits, direct shipment of products to the homes of participants.
About Tufts CSDD & Scimitar
The Tufts Center for the Study of Drug Development (Tufts CSDD) is an internationally recognized nonprofit academic research center. Its mission is to provide strategic information to help stakeholders throughout the drug development enterprise improve the quality, performance, and efficacy of pharmaceutical innovation. For more than 45 years, Tufts CSDD has conducted scholarly analyses that address the economic, scientific, legal, management, and political issues that affect the development and regulation of human therapeutics.
To achieve its mission, Tufts CSDD conducts primary and secondary research, maintains and develops robust datasets, and interacts regularly with a global community of stakeholders. Tufts CSDD is funded primarily by research grants from pharmaceutical and biotechnology firms, and companies that provide services and solutions to the research-based industry – including trade associations, contract service providers and technology firms, government agencies, and foundations.
Scimitar Inc. is an experienced life sciences management consulting firm that provides strategic industry insights and high-return solutions. Founded in 2007, Scimitar sought to fill a void in the life sciences industries. Scimitar founders realized that life sciences companies were relatively early in their operational maturity when compared to long-established product development, manufacturing, and supply chain industries such as automotive or consumer goods.
Scimitar Inc. brings best practices and innovative solutions to life sciences organizations via cutting-edge consulting in a collaborative effort to increase client profits and reinvestment in life-saving research, ultimately improving the well-being of patients everywhere.
From research through commercial, Scimitar drives operational transformations and strategic initiatives across the drug product lifecycle.
Contact Us
Interested in joining or learning more about the PACT Consortium?
Please complete the form or send an email to csdd@tufts.edu and we will promptly follow up. Thank you.
