Our group engages in areas of bioengineering related to cell engineering and bioprocessing for improving human health. Toward this goal, experimental approaches in tandem with computational methods are employed.
Stem cell engineering
Stem cells can serve as inexhaustible source of functional cells for addressing life-altering pathologies including diabetes and cardiac diseases. Realization of this potential depends on the production of therapeutically useful stem cell derivatives in clinically relevant quantities. Our work in this area focuses on addressing facets of stem cell bioprocessing including the effects of the bioreactor environment and metabolism on the physiology of stem cells during propagation or directed specification to cells such as pancreatic endocrine cells.
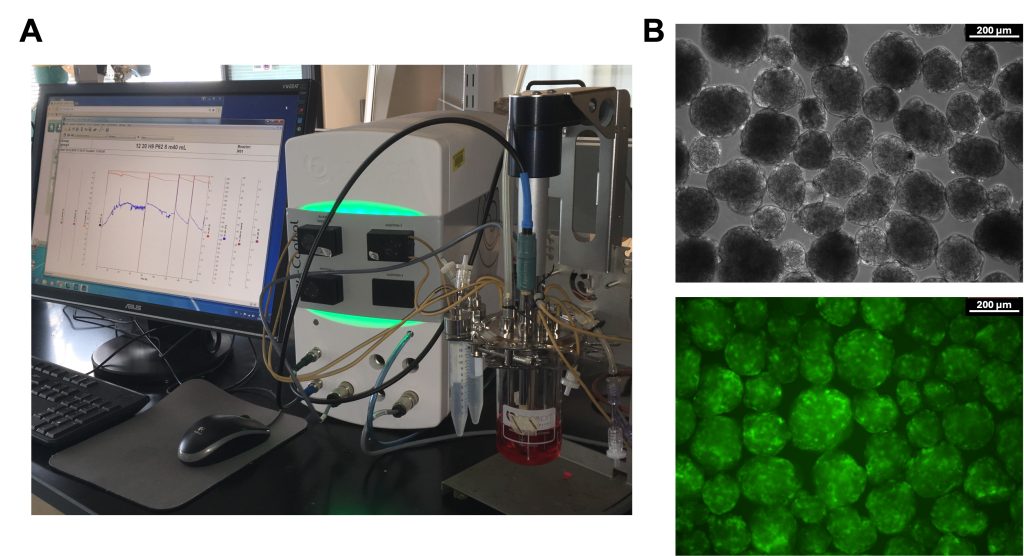
This work is coupled with multiscale modeling and digital twins capturing the heterogeneous nature of stem cell ensembles. Elucidation of the role of the exhibited heterogeneity on cell and tissue fate determination and function is key to developing efficient strategies for engineering stem cell products.
Optogenetic control of cellular function
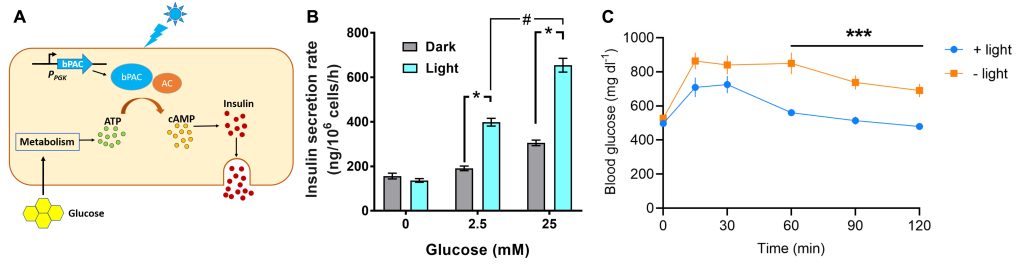
We employ optogenetics relying on the use of light-activated proteins to control specific cell functions such as the contractility of cardiomyocytes and the secretion of hormones by pancreatic endocrine cells. This requires the careful design and embedding of synthetic circuits in the cellular machinery for precise control of the desired function without detrimental side effects.
To enhance the amount of insulin secreted by pancreatic β-cells in a drug-free manner, our group has engineered rodent and human β-cells expressing a bacterial photoactivatable adenylyl cyclase (PAC). Activation of PAC with light augments the glucose-stimulated release of insulin compared to cells without PAC. These findings open prospects of engineering systems which allow drug-free control of blood glucose, for example, in diabetic patients. Pancreatic β-cells expressing PACs can be integrated in a bioartificial pancreas device along with an appropriate light source for stimulation and a glucose sensor, enabling future diabetes therapies.
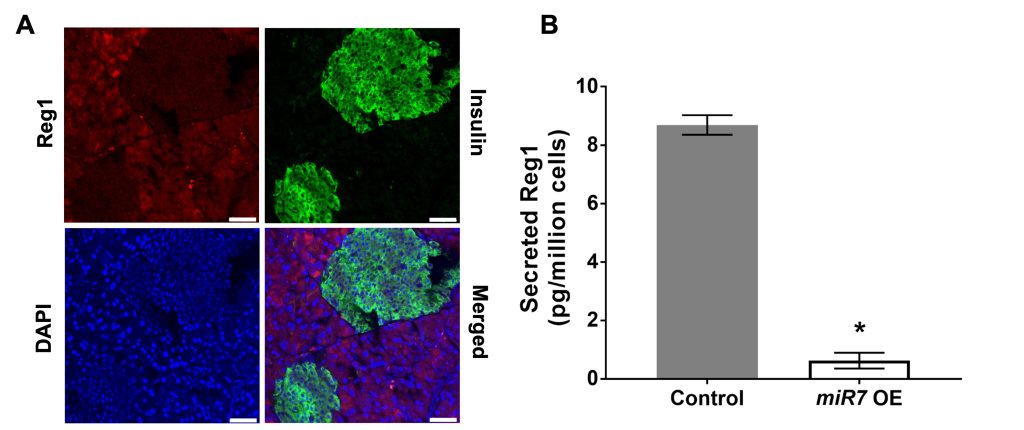
Reg protein biology
Regenerating islet-derived (Reg) proteins were discovered in the pancreatic secretions of patients with chronic calcifying pancreatitis. However, the presence of Reg proteins has been confirmed both in pancreatic and extra-pancreatic tissues particularly under diverse pathological conditions such as diabetes and cancer. Reg proteins have been postulated to promote proliferation and differentiation while preventing apoptosis, but there is very little known about their exact role(s) almost 50 years after their discovery. Our research focuses on gaining a deeper understanding of the physiological functions of Reg proteins and underlying mechanisms in normal tissue homeostasis and disease.
Adeno-associated virus for pancreatic cell engineering
Adeno-associated viruses (AAVs) are currently being leveraged as advantageous gene delivery vehicles in emerging therapies given the AAVs’ lower immunogenicity compared to other viral vectors, episomal long-term expression of transgenes, and a wide range of tropism. We are primarily interested in utilizing AAVs for introducing genes to pancreatic cells. The AAV serotypes 2, 6 and 9 target distinct cell surface receptors offering opportunities for enhancing the virus-cell interactions for higher transduction efficiency. The resulting strategies can aid the engineering of pancreatic cells, particularly in the context of pathologies such as diabetes and pancreatic cancer.

