Research
Transgenerational Epigenetic Inheritance
Transgenerational epigenetic inheritance—the passing of environmental influences across generations—is a growing area of research with profound implications. Evidence from clinical and preclinical studies suggests that drug exposure in one generation can increase the risk of substance use disorders in subsequent offspring. This issue is particularly pressing in the context of the ongoing opioid epidemic, where adolescent exposure to opioids is widespread.
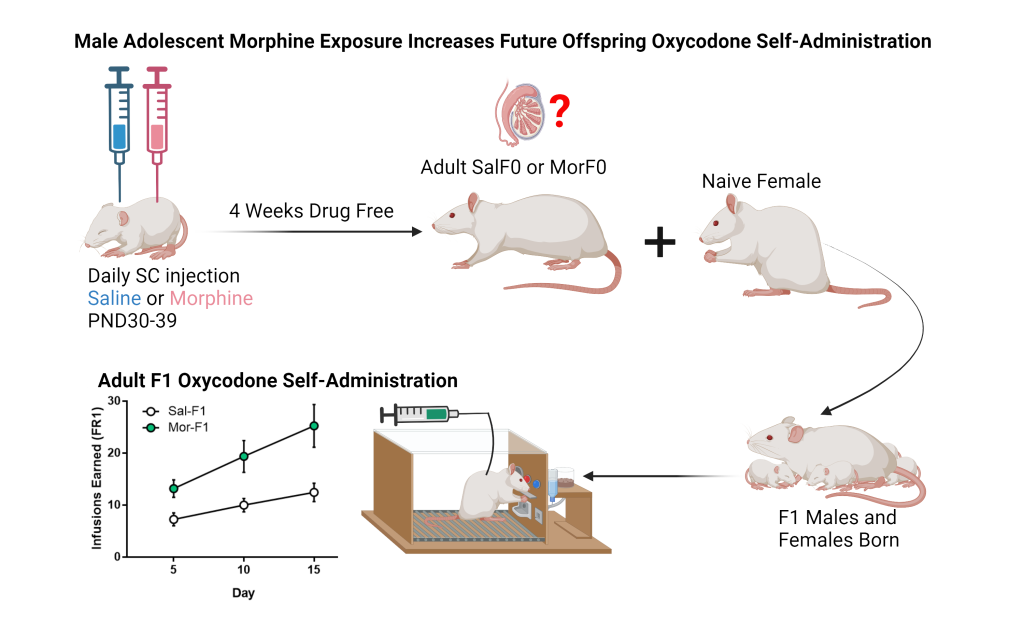
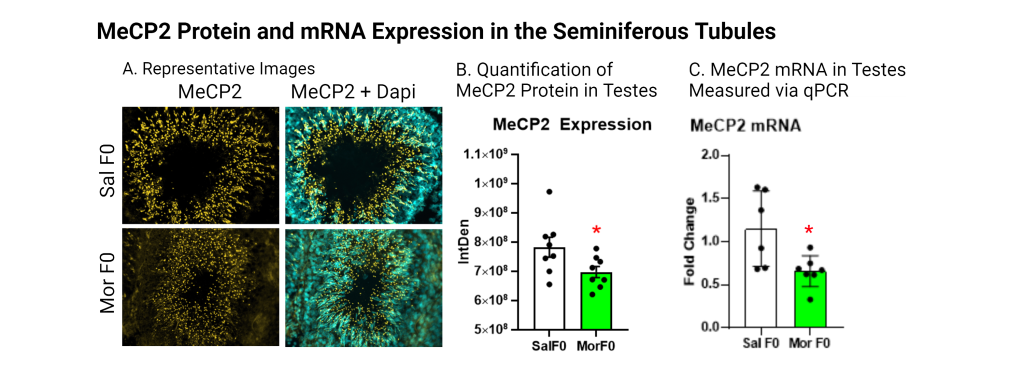
Our research has revealed that male rats exposed to morphine during adolescence produce offspring that demonstrate increased oxycodone self-administration. Building on this finding, we are investigating how opioid exposure in fathers affects offspring and grand-offspring through epigenetic modifications in testes and sperm. For example, we have noted differences in histone acetylation and MeCP2 levels in seminiferous tubules. In particular, we focus on let-7 microRNAs (miRNAs), a family of molecules that regulate the mu-opioid receptor (MOR), which is critical to opioid tolerance and reward.
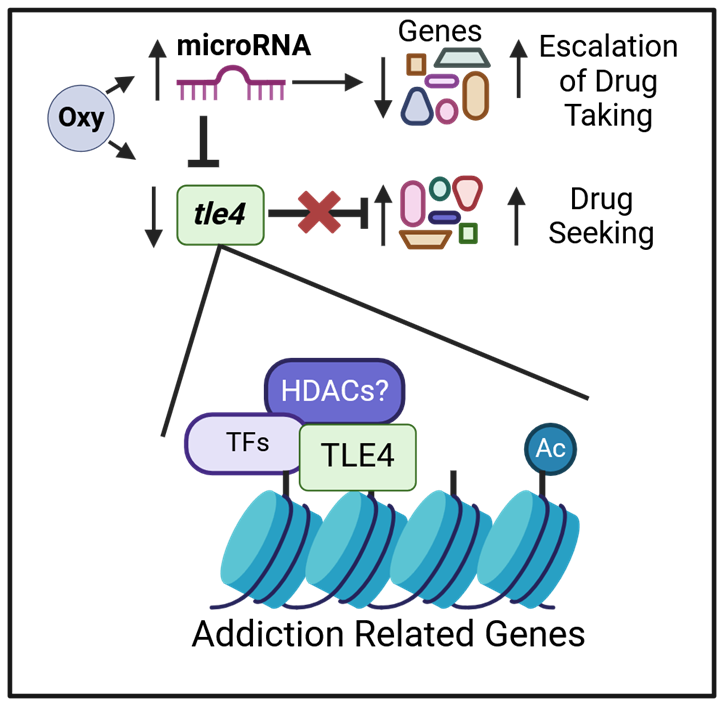
We have found that opioid exposure in male rats leads to persistent upregulation of let-7 in their sperm, even after prolonged abstinence. This upregulation likely reduces MOR levels in the offspring’s brain, potentially increasing vulnerability to opioid addiction-like behaviors. Current experiments utilize advanced approaches, including let-7 inhibition and artificial insemination, to determine the role of sperm let-7 in modulating MOR expression and addiction behaviors across two generations.
This research has significant implications for understanding how substance use disorders may be inherited non-genetically, offering potential targets for intervention and prevention strategies in the face of the opioid crisis.
The role of miRNAs and epigenetics in orchestrating sustained neural changes that maintain an addiction-like state.
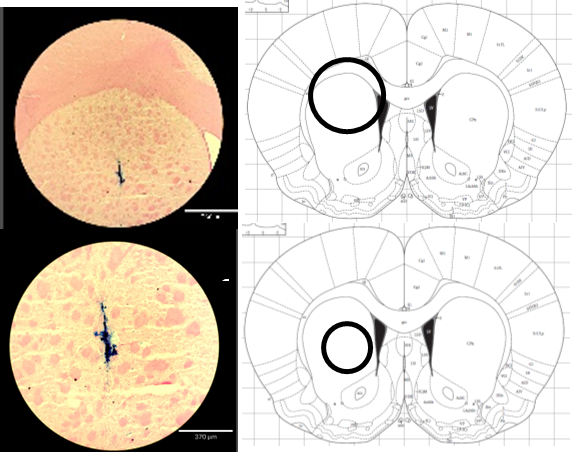
One of the most challenging aspects of opioid use disorder (OUD) is remaining abstinent. Indeed, there is an exceedingly high rate of recidivism in OUD with estimates between 50-70% of individuals experiencing relapse events. These studies within our lab are aimed at examining the role of miRNAs in altering the transcription of many mRNAs and maintaining an addiction-like brain state leading to relapse. Using a preclinical model, we use a combination of short and long-access self-administration combined with full transcriptome sequencing of both mRNAs and miRNAs. Working together with computational biologists (like Rebecca Batorsky), we are developing models (including weighted correlation network analysis; WGCNA) by which we can determine miRNAs that may be involved in widespread and subtle changes in gene expression that maintain altered brain states that increase chances of reinstatement. We will then use viral expression of specific miRNAs within limbic brain regions to test if we can shift the brain to or from an addiction-like state to determine if the miRNAs are playing a causal role in maintaining long-term changes in gene expression underlying addiction
Novel Therapeutic Development for Opioid Use Disorder
Novel therapies are urgently needed to help individuals stop the cycle of substance use disorder. One such therapy is non-invasive magnetic mechanical stimulation. Based on previous work demonstrating effectiveness of deep brain stimulation (DBS) to attenuate reinstatement of drug-seeking behavior, we are now testing a novel type of DBS that is less invasive. This therapeutic strategy involves inhaling magnetic nanoparticles intranasally. A large external magnet then pulls the particles through the cribriform plate along the olfactory nerve and into the brain parenchyma. They can then be precisely moved to appropriate deep brain structures. Once in place, a wearable magnetic device would oscillate the magnetic particles to mechanically stimulate nearby neurons. Our lab is testing if external magnetic stimulation of nano-scale magnetic rods situated in the nucleus accumbens can decrease reinstatement. Collaborators at Weinberg Medical Physics are testing ultra-fast magnetic resonance imaging (MRI) and nanoparticle drivers’ capacity to deliver the MNPs via intranasal inhalation to precise deep brain structures. This novel delivery method could potentially allow the delivery of MNPs in an outpatient setting without the need for invasive surgery.
Using DREADDS to measure sex differences in development and expression of locomotor sensitization to oxycodone.
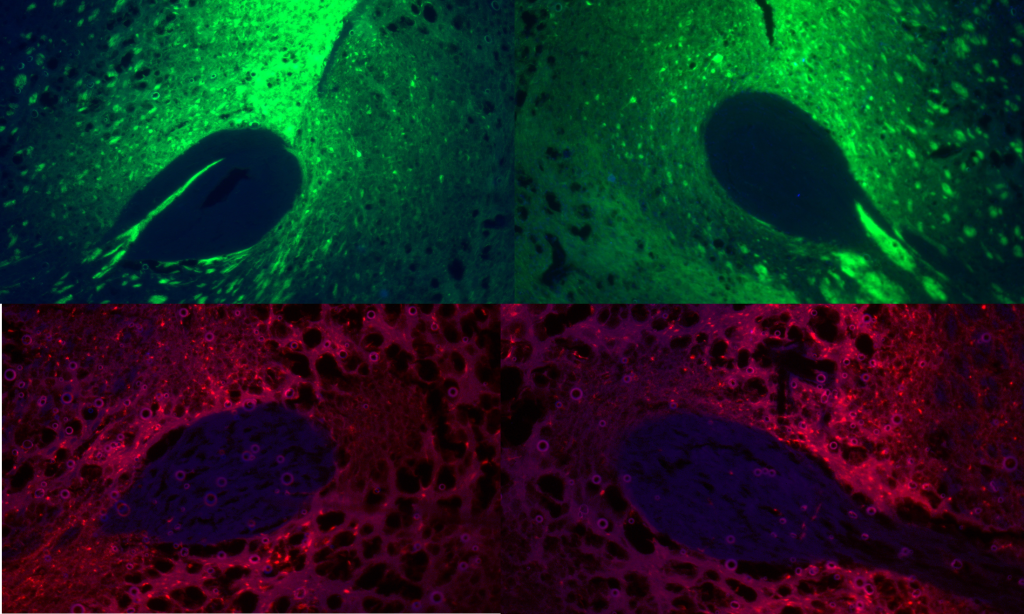
Oxycodone remains the most abused prescription opioid and often leads to the development of illicit opioid use such as heroin or fentanyl. Yet, there remains a paucity of literature that examines brain changes associated with oxycodone, particularly in females. In this set of experiments, we are exploring sex differences in oxycodone sensitization. Using designer receptors exclusively activated by designer drugs (DREADDS) we are testing which brain regions are involved in both the development and expression of sensitization to oxycodone.
