LEDs
In 2014 the Nobel prize for physics was awarded to a research team who invented a new kind of LED diode. Light emitting diodes (LEDs) have been around for ages, so what makes this new variant so special? This article discusses how LEDs work and what makes this light-emitting technology so special.
LEDs
Light Emitting Diodes – or LEDS for short – are a variety of semiconductor that generates light when current passes through it. First invented in 1962, LEDs have always been good as indication lights but for a long time their low levels or brightness kept them away from from traditional lighting applications. Due to recent developments in the technology they have started replacing incandescent light bulbs in everyday use. This article will introduce the LED, discuss the technology, applications, and why a certain LED was worthy of the 2014 Nobel prize in physics.
Light has always been a necessity for life as we know it. For most creatures, early humans included, light came from the sun. The discovery of fire, an invention of mythological proportions brought about humanity’s first artificial and controllable light source, illuminating caves, little houses on the prairie, and big Victorian manors. The next milestone didn’t come for thousands of years at the invention of the incandescent light bulb. The incandescent bulb would reign supreme for about a century until fluorescent bulbs were invented, and then, finally, the led bulb.
LED light bulbs have been gaining immense popularity in the past few years because they can be just as bright as incandescents, but with higher efficiency and a longer expected lifetime.
But there is a half-century unaccounted for during which LEDs existed but LED light bulbs did not. In the first few years LEDs could do no more than generate tiny, barely visible amounts of light; the idea of illuminating any real amount of space was completely infeasible. In general, LED bulbs lacked price efficiency and energy efficiency. The metric for measuring the efficiency of a light bulb is in lm/W, or lumens per watt. A lumen is a measure of light brightness over an area, and a watt is a measure of energy consumption. In layman’s terms, light efficiency is (brightness / energy).
Figure 1
Types of LEDs. Source: Author.
Electro-Magnetism
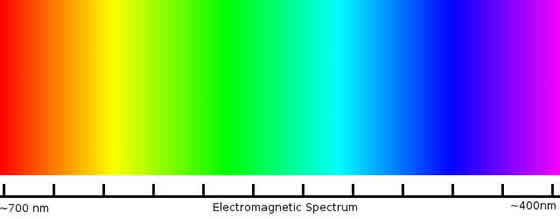
Before we begin talking about light emitting diodes it would do us well to discuss the physics behind light itself. Light is a kind of electromagnetic wave. The everyday light that we think of as “Light” is actually only a narrow band on the electromagnetic spectrum. Other kinds of electromagnetic waves are radio waves, microwaves, x-rays and gamma rays. All of these are forms of light, but they are outside the visible spectrum so we do not see them as traditional light. The wavelength of the light will determine what kind of wave it is, and with in the visible light spectrum it will also determine the color of the light: As seen in Figure 2, Light with a wavelength of approximately 700nm will appear red, and light with a wavelength of approximately 400nm will appear blue or purple.
Figure 2
The Electromagnetic Spectrum of Visible Light.
This means that if we have light of a specific wavelength, we will see it only in that color. Everyday objects have color because they reflect certain wavelengths of light more than others. A green apple reflects green light, a red fire hydrant reflects red light. If one shines red light on a green apple, the light is absorbed instead of reflected. White light is a composition of every color of light. When one shines white light on a green apple the green is reflected but the other colors are all absorbed. Therefore, the light coming off of the apple is green and the apple appears green to the human eye.
Light Emission and the Bohr Model
There is no specific wavelength of visible light with the color white. This is because it is possible to generate different colors of light by combining multiple different wavelengths of light. White light is actually the equal combination of every wavelength of visible light. A typical light bulb with a whitish-yellow light happens when the light emits most colors of light, but emits more light with a yellow wavelength than others. The typical emission spectrum of a household light bulb can be seen below in Table 1. Our sun is also strong around the yellow spectrum, and other stars that appear red or blue have their corresponding color intensities.
LEDs, on the other hand, only emit a handful of wavelengths of light. This is because the materials that LEDs are made of, unlike stars or incandescents, are only able to emit a certain few wavelengths when electrically excited. As Table 1 shows, different LEDs have different emission spectra: each with a very thin emission spectrum. LEDs only emit one color of light not because they have colored plastic over white light, but because they only emit light at a single wavelength.
Table 1
EM Spectra of Various Colors.
The Bohr model of the atom explains how atoms emit various lights. A typical atom has some amount of both electrons and protons: typically these counts are are equal and they define the atom’s behavior. Typically, the electrons come in shells: each with a different associated energy.
When the atom receives energy, (electrical, thermal, mechanical, etc) electrons can move out to higher energy shells. Then, when the electrons move back down to lower energy shells they release a specific wavelength of light. This is an oversimplification of the behavior, but the important takeaway is that there are only a few specific frequencies of light that can be emitted by atoms.
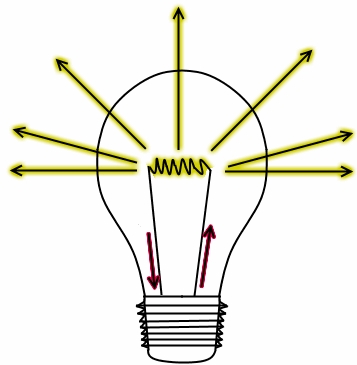
The typical operation of an incandescent light bulb can be seen in Figure 3. When electricity passes through a coil of a certain material, the electrons crash into the atoms of the material, exciting the atoms. Then, as the atoms lose energy, they emit light. Incandescent lights force electrons to higher energy levels, then when the electrons fall back down the light is emitted. However, incandescent lights brute force this process: there is no control over how the energy is absorbed and released. This means that the color of the light coming out of an incandescent bulb is essentially random, and the composition of all those random colors is the white light we see from those bulbs.
Figure 3
Operation of an Incandescent.
LED Operation
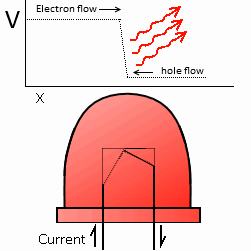
LEDs generate light differently than incandescents. While incandescent filaments randomly and haphazardly throw out light of many wavelengths, LEDs go about the process in a much more regulated and refined way. Through a process known as recombination, electrons flowing in one direction combine with holes – a current that flows via the absence of electrons – flowing in the other direction. When these two currents meet, the electrons and the holes recombine, restoring the electrical neutrality of the conductor. The electrons will be in a higher energy level than the holes though, so as the electron falls down it emits exactly the right wavelength of light desired.
Figure 4
LED Operation.
Different LED colors are generated by controlling how far the electron falls into the hole it combines with. Different wavelengths of light are produced by different voltage potentials between the two currents. In general, the color and intensity of the light produced depends on the chemical makeup of the semiconductor. Infrared emitting LEDs, which emit a wavelength slightly longer than visible red light, were the first LEDs ever invented back in 1962. (Hall et al.) Infrared LEDs were first made of Gallium Arsenide (GaAs. By adding phosphorous to make GaP or Gallium Phosphide diodes, all sorts of other colors such as red green and yellow. A lot of work has gone into discovering new semiconductors that emit colors brighter or more efficiently, but for a very long time researchers struggled to make blue LEDs, let alone affordable blue light.
Blue LEDs, White LEDs and Spectrum Adjustment
For a long time, scientists and researchers chased the holy grail that was the fabrication of blue or short wavelength LEDs. Green and red LEDs had already existed, but to build a white LED light blue would also be needed. The problem was that while blue LEDs could be made out of GaN, or Gallium Nitride, growing a pure crystalline lattice of the material proved exceptionally difficult. It wasn’t until 1989 that the first blue led appeared in research labs thanks to completely new fabrication techniques that introduced new levels of precision and control to the process. By 1992, the addition of Aluminum Gallium Nitride (AlGaN) and layers of Indium Gallium Nitride (GaInN) improved light efficiency tenfold. Since then, efficiency has improved tenfold again. For their efforts in this development, Isamu Akasaki, Hiroshi Amano and Shuji Nakamura were awarded the 2014 Nobel Prize in physics.
Alone, a blue LED does not mean much. These days, pure blue leds are mostly used for power buttons or other decals and indicators. The invention of the blue LED was not important because it is blue, it is important because it enabled white colored LED light. When a red LED. A green LED and a blue LED are put in close proximity to each other and the light is diffused, the resulting color is a white led. And if only one or two of the LEDs are on, any color of light can be created. The other method of creating white light is known as spectrum adjustment.
Spectrum adjustment happens when light is absorbed and then re-emitted again. By doing so, the color and appearance of the light can be altered. There is a caveat, however, that the light can only be shifted to larger wavelengths. If a material were able to absorb a long wavelength of light and emit the same amount of a shorter wavelength, this would violate the conservation of energy. This is why it was not possible to generate blue light from other diodes. However, the reverse process does in fact work: if a blue diode is passed thorough a yellow phosphor, the blue and yellow combine into a white light well suited for everyday tasks.
LEDs in Everyday Applications
Before they were available as household lights, LEDs served as indicators in devices and continue to to this day. LEDs as indicators are an essential and powerful learning tool for beginner electronics: They provide immediate feedback as to if a circuit is properly working. In fact, one ofthe first programs that anyone learning the Arduino platform will write is called “blink” and all that it does is blink an LED.
Beyond homes and headlights, LED lamps have started moving into city planning offices. Due to their efficiency and long lifetimes, LED street lamps have begun phasing into many cities across the globe, including Somerville, Arlington, and many other towns in Massachusetts. LEDs as lights is where the big breakthroughs have been in the past few years, so the primary question now is how many devices can we integrate LEDs into and how quickly can we integrate these bew lighting forms into society’s infrastructure.
Now that LEDs have surpassed traditional lighting methods in terms of lifetime and efficiency, they have opened the floodgates of efficiency to other light-using technologies. LCD televisions, for example, once required large fluorescent lights that would be filtered through an LDC display to produce an image on screen. By using an array of white LEDs instead, not only is power usage drastically lowered, but it’s even possible to make higher contrast screens, by actually dimming the light in certain areas instead of just filtering it out.
On the subject of screens, some screens are made entirely of OLEDs – or organic LEDs. Rather than standard LEDs which typically have a small light-emitting area, OLEDs are made of many planar sheets that form a full surface capable of light emission. OLEDs reduce the thickness of devices and allow screens that are made of just LEDs – no LCD filter required. While the technologies and chemical compositions are different than normal LEDs, they are still relevant as one of the next big frontiers of LEDs.
Conclusion
To quote the Nobel Prize article on the invention: “Incandescent light bulbs had lit the 20th century; the 21st century will be lit by LED lamps.“ Compared to Incandescents lights, LEDs are better in almost every way. They have a smaller energy footprint, they have a longer lifespan and they can be put into a smaller package. Lights account for 19% of the energy that the whole world uses, and incandescent lights are only 5% effective. If all of that energy went into incandescent lights, that would mean over 18% of the world’s energy is immediately lost as heat. That’s a huge amount. LEDs are more expensive to create and manufacture but they pay for themselves in energy savings in no time at all.
New technology often struggles with mass adaption. However, thanks to the short lifespan of incandescent bulbs, the process of phasing them out is much easier than it could be. As energy usage rises worldwide, the integration of this technology could be key for butting back. Blue LEDs are not only for lighting, they also appear in the new LED and OLED based screens for televisions and phones. Upon closer inspection of many devices, LEDs much more prevalent and much more significant to everyday technology than one might think.
Bibliography
- Akasaki, I. (2007). Key inventions in the history of nitride-based blue LED and LD 300(1). DOI: 10.1016/j.jcrysgro.2006.10.259
- Aspense, D.E., Studna, A.A. (1983). Dielectric functions and optical parameters of Si, Ge, GaP, GaAs, GaSb, InP, InAs, and InSb from 1.5 to 6.0 eV. Phys. Rev. B 27(2). DOI: 10.1103/PhysRevB.27.985
- Bergstrom, L., Delsing, P., L’Huillier, A., Inganas, O., Rose, J., Blue LEDs – Filling the world with new light. Retrieved from the Royal Swedish Academy of Sciences
- Hall, R.N., Fenner, G.E., Kingsley, J.D., Soltys, T.J., Carlson, R.O. ( ). Coherent Light Emission from GaAs Junctions. Phys. Rev. Letters 9(9). DOI: 10.1103/PhysRevLett.9.366
- Kalinowsk, J., Di Marco, P., Fattori, V., Giuletti, L., Cocchi, M., (1998). Voltage-induced evolution of emission spectra in organic light-emitting diodes. Journal of Applied
Physics, 4242-4242. DOI: 10.1063/1.367181 - Kamtekarm, K., Monkman, A., Bryce, M., (2009) Recent Advances in White Organic Light-Emitting Materials and Devices (WOLEDs). Adv. Mater. 2010, 22, 572–582. DOI: 10.1002/adma.200902148
- Park, J., Lee J.H., Raju, S.R., Moon, B.K., Jeong, J.H., Choi, B.C., Kim, J.H. (2014). Ceramics International, 40(4). DOI: 10.1016/j.ceramint.2013.11.007
- Sato, T., Imai, M.(2002). Characteristics of Nitrogen-Doped GaAsP Light-Emitting Diodes. Jpn. J. Appl. Phys. DOI: 10.1143/JJAP.41.5995