My Sites
Log in to create or edit your sites.
IMPORTANT SERVICE ANNOUNCEMENT: Content freeze 7am June 19th until 7am June 23rd. Read more here.
Need Help? Email edtech@tufts.edu
Site-Wide Activity
-
esefik01 wrote a new post on the site Emotion, Brain, & Behavior Laboratory 10 years, 7 months ago
Schizophrenia as Split Personality: Jekyll and Hyde?
Dr. Henry Jekyll was a “large, well-made, smooth-faced man of fifty with something of a stylish cast”, who occasionally felt like he was battling between the good and the evil within himself, thus leading to the struggle between his dual personalities of Jekyll and Edward Hyde. He created a potion, in an attempt to mask this hidden evil within his personality. In doing so, Jekyll accidentally transformed into Mr. Hyde, a hideous, evil creature without compassion or remorse.
The strange case of Dr. Jekyll and Mr. Hyde is a well-known example of a psychiatric disorder, commonly known as split personality. Clinically known as dissociative identity disorder, split personality is a mental disorder that is characterized by “at least two distinct and enduring identities or dissociated personality states that alternately control a person’s behavior”. It is also accompanied by memory impairment for important information that can’t be explained by ordinary forgetfulness. Doesn’t really sound like a psychiatric symptom that patients with schizophrenia suffer from, does it?

Does schizophrenia mean split personality? Contrary to public perception, people with schizophrenia do not have split personalities.
Unfortunately, there is a common misconception in society that schizophrenia is the same as a “split personality”. The word “schizo” does indeed mean “split”. However, Eugen Bleuler, who coined the term schizophrenia in the 1920s, was describing the rupture in the person’s thinking process and emotional response when he coined the term. In today’s blog post, I will talk about this “split” in schizophrenia, not as a Jekyll and Hyde syndrome, but in terms of the discrepancies between a schizophrenic patient’s emotional experience and facial expressiveness.
In 1993 Kring et al. conducted a groundbreaking research study on the disjunction among emotional response domains in schizophrenic patients. This was a time when affective features of schizophrenia had only begun to receive empirical attention. Kring et al. (1993) examined the relationship between emotional experience and expression in a sample of medication-free schizophrenics. The results of this study indicated that compared to healthy control subjects, “people with schizophrenia were much less facially expressive of both positive and negative emotions during emotion-eliciting films. However, they reported experiencing as much positive and negative emotion as the healthy control subjects”. The findings of Kring et al’s (1993) study were crucial in proving that clinical blunting ratings of facial expressivity (flat affect) in schizophrenia do not reflect diminished emotional experience in this population.
Another study focusing on this disjunction was conducted by Aghevli et al. in 2003. They wanted to investigate whether this disjunction between emotional experience and expression in schizophrenia extends to the interpersonal domain. Previous literature had focused solely on a number of non-social stimuli such as film clips while examining this discrepancy. Aghevli et al. (2003) compared patients with schizophrenia and non-psychiatric controls during a social role-play. The results indicated that “patients were significantly less expressive than controls but the two groups reported equal levels of emotional experience”. These results extended the prior findings of the expression/experience disjunction in schizophrenia to a social domain. This way, their findings made this disjunction more relevant to understanding poor social functioning and interpersonal interaction difficulties experienced by people with schizophrenia.
After reading about these thought-provoking empirical findings, the question that arises: What are the neural substrates of this expression/experience disjunction in a brain suffering from schizophrenia?
 A literature review will show that there is no MRI study that specifically investigates the neuronal dysfunctions or abnormalities that could potentially be the primary cause of this experience/emotion dissociation in schizophrenia. However, there are a number of more general fMRI studies that look at the dysfunction of emotional brain systems in schizophrenia with a focus on amygdala abnormalities. Aleman et al.’s 2005 study hypothesizes that specifically, “a lesion to the amygdala in combination with reduced interconnectivity with the prefrontal cortex potentially give rise to reduced emotional expression (affective flattening) and emotion recognition deficits”. Such abnormalities in the structure of the amygdala as well as disconnections in frontolimbic pathways could potentially be one of the main causes of such expression/experience dissociation in schizophrenics.
A literature review will show that there is no MRI study that specifically investigates the neuronal dysfunctions or abnormalities that could potentially be the primary cause of this experience/emotion dissociation in schizophrenia. However, there are a number of more general fMRI studies that look at the dysfunction of emotional brain systems in schizophrenia with a focus on amygdala abnormalities. Aleman et al.’s 2005 study hypothesizes that specifically, “a lesion to the amygdala in combination with reduced interconnectivity with the prefrontal cortex potentially give rise to reduced emotional expression (affective flattening) and emotion recognition deficits”. Such abnormalities in the structure of the amygdala as well as disconnections in frontolimbic pathways could potentially be one of the main causes of such expression/experience dissociation in schizophrenics.To conclude, currently emotional processing in people with schizophrenia is an exciting and ever-growing area of research. Functional and structural neuroimaging has gained remarkable popularity in localizing brain-areas related to deficits in emotional and cognitive processing. The clinical implications of such findings are crucial in that they can guide clinicians in setting more effective treatment plans for patients with schizophrenia, that not only address the positive symptoms of the disease but also the negative symptoms that are based on emotion deficits such as the expression/experience disjunction. Tune in next week to learn more about the ever-growing gap between treatment priorities and unmet needs of the patients in terms of negative symptoms!
Sources:
Aleman A, Kahn RS. Strange feelings: Do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Progress in Neurobiology (2005) 77 (5): 283–29.
Aghevli, M. A., Blanchard J. J., Horan W. P. The Expression and Experience of Emotion in Schizophrenia: A Study of Social Interactions (2003) 119:261-70.
Kring A. M, Kerr S.L., Smith D.A., Neale J.M. Flat Affect in Schizophrenia does not Reflect Diminished Subjective Experience of Emotion. Journal of Abnormal Psychology (1993) 102(4): 507-17.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. (2013). Washington, D.C: American Psychiatric Association.
Ashok, A.H., Baugh J., Yeragani, V. K. Paul Eugen Bleuler and the Origin of the Term Schizophrenia. Indian Journal of Psychiatry (2012) 54(1):95-96.
Stevenson, Robert Louis. The Strange Case of Dr. Jekyll and Mr. Hyde. NewYork: Dover Publications Inc., 1991. Print.Schizophrenia. Indian Journal of Psychiatry (2012) 54(1):95-96.
Fig 1. http://skeptikai.com/wp-content/uploads/2012/06/WEBsplitpersonality.jpg
Fig 2. http://photos2.demandstudios.com/dm-resize/photos.demandstudios.com%2Fgetty%2Farticle%2F88%2F112%2F87577833_XS.jpg?w=400&h=10000&keep_ratio=1
Fig 3. http://static.guim.co.uk/sys-images/Guardian/Pix/pictures/2010/04/08/Brain-scan.jpg -
Neil Patel is attending Seminar III. 10 years, 7 months ago
-
William Brown is attending Seminar IV. 10 years, 7 months ago
-
William Brown is attending Seminar III. 10 years, 7 months ago
-
Lindsay A. Hinzman wrote a new post on the site Emotion, Brain, & Behavior Laboratory 10 years, 7 months ago
-
Katharina S. Fung is attending Seminar IV. 10 years, 7 months ago
-
Amy M. Smith wrote a new post on the site Emotion, Brain, & Behavior Laboratory 10 years, 7 months ago
-
Lisa M. Cerrato wrote a new post on the site Perseus Digital Library Updates 10 years, 7 months ago
The Perseids team is delighted to announce a grant from the Samuel H. Kress Foundation (http://www.kressfoundation.org/) for the Digital Milliet. The Samuel H. Kress Foundation devotes its resources to adv […]
-
Rebecca Rago wrote a new post on the site Emotion, Brain, & Behavior Laboratory 10 years, 7 months ago
Welcome back! The past blog posts have focused on the general role of our senses and our emotions, and the specific role of our visual system in emotion. This post’s focus will be on the auditory system. Let’s start with an exercise.
Listen to this: . Do you feel anxious? Alert? Nervous? What about this: ? Do you feel peaceful, relaxed, and at ease? Our auditory system can trigger different emotions. This blog post will explore the ways our auditory system can aid us in emotional recognition and processing. It will also examine how deafness affects emotions.
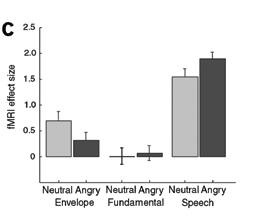
The differences in STS activation when listening to emotional words versus neutral words or sounds of similar frequency or amplitude. (Grandjean et al., 2005)
One of the main ways which we interact with other people is through speech. When we speak, we often times display emotions in our words or voices. Someone’s words may become clipped and loud in anger, or may seem to wobble when they’re sad. A study by Grandjean et al. (2005) examined how these vocal differences affect the brain. Participants listened to angry and neutral words or just neutral words. They also listened to sounds of similar frequencies or amplitudes as controls. Participants who listened to the angry words had an increased activation in their right superior temporal sulcus (STS) of the auditory cortex. A study by Ethofer et al. (2006) supplemented these findings. Participants listened to words spoken in either angry, neutral, or happy tones. They rated the intensity of each word while in an fMRI. They too found increased activation for angry words in both sides of the STS, but stronger activation on the right. Interestingly, this activation was found even though participants were just rating intensity. These findings tell us that the auditory cortex is very involved in identifying voices as bearing some emotion. This identifying of emotion may take place in conjunction with just hearing and deciphering the words. It also may be done regardless of what else is being focused on (Ethofer et al., 2006). This makes sense, it is a good thing to understand the emotions of a person you are communicating with. Sometimes, the person may not directly tell you. Other times, it may be useful to help empathize with them. Brain activation in terms of emotions can change based upon the emotion in question.

Which face would you most want to help? You’d probably want to help the sad face more. Our auditory cortex aids this by being more in tune to sad tones of voice (Buchanan et al., 2000)
Buchanan et. al (2000) found that increased STS activity varies based upon emotions. Participants listened to words that were said angrily, happily, neutrally, or sadly while in an fMRI scanner. They pressed a button when they heard a target word was heard or a word was spoken in a target emotion. Participants were more successful in emotional identification. In addition, greater right anterior auditory cortex activation was seen in the emotional tasks. This may indicate that our auditory systems process emotions before content (the actual word). Further, the right anterior middle frontal gyrus had more activation for sad words. This shows that sad intonations may alert us more than happy ones. This may be due to the fact that when someone is sad, we may be more in tune to them. Think about it. You are more likely to help and want to alter a sad person’s state (back to at least neutral) than change a happy person’s mood. How does this change for those who have impaired hearing? Research shows that emotional processing changes dramatically for the deaf.

Examples of “happy” faces used as stimuli by Ludlow et al. (2010).
Ludlow et al. (2010) aimed to see how emotional processing is impacted for deaf children. Deaf children and normal hearing children participated in their study. Participants viewed faces and categorized the emotions displayed. Faces were either human, a human like cartoon, or a cartoon. These faces were oriented upright or upside down. Both groups were equal on the two cartoon faces, and both groups performed worse on the upside down faces. Deaf participants, however, made more errors overall and had significantly worse performance. A study by Dyck et al. (2004) compared the emotional recognition abilities of hearing impaired and normal hearing children. He used four tests including: “Fluid Emotions Test” which looks at one’s accuracy in classifying changing emotions and “Emotion Vocabulary Test” which looks at ability to define and describe emotional words. In all five tests, hearing impaired children performed worse. This implies that substantial emotional learning comes from what we hear. It allows us not only to process the emotions in someone’s voice, but also how we utilize and apply knowledge to emotions.
It just makes sense that our sense of hearing (pun intended) underlies so much of our learning in emotion. Hearing is one of the first senses we really grasp. Babies are said to be in tune to the voices from early on. Hearing allows us to take in information about the world before anything else. You sometimes hear aspects of a situation before you arrive at the scene. As seen in this post, our auditory cortex increases in response to emotional tones. Those who are deaf have severely impacted emotional understanding. Being able to understand emotions from hearing, is especially important now where conversations may take place without any direct contact such as via phone. Next post, we will look at how our gustatory and olfactory systems can play into our emotion. Stay tuned!
References
Buchanan, T. W., Lutz, K., Mirzazade, S., Specht, K., Shah, N. J., Zilles, K., & Jäncke, L. (2000). Recognition of emotional prosody and verbal components of spoken language: an fMRI study. Cognitive Brain Research, 9(3), 227-238.Dyck, M. J., Farrugia, C., Shochet, I. M., & Holmes‐Brown, M. (2004). Emotion recognition/understanding ability in hearing or vision‐impaired children: do sounds, sights, or words make the difference?. Journal of Child Psychology and Psychiatry, 45(4), 789-800.
Ethofer, T., Anders, S., Wiethoff, S., Erb, M., Herbert, C., Saur, R., … & Wildgruber, D. (2006). Effects of prosodic emotional intensity on activation of associative auditory cortex. Neuroreport, 17(3), 249-253.
Grandjean, D., Sander, D., Pourtois, G., Schwartz, S., Seghier, M. L., Scherer, K. R., & Vuilleumier, P. (2005). The voices of wrath: brain responses to angry prosody in meaningless speech. Nature neuroscience, 8(2), 145-146.
Ludlow, A., Heaton, P., Rosset, D., Hills, P., & Deruelle, C. (2010). Emotion recognition in children with profound and severe deafness: Do they have a deficit in perceptual processing?. Journal of clinical and experimental neuropsychology, 32(9), 923-928.
-
Prabhdeep Singh is attending Seminar V. 10 years, 7 months ago
-
Michelle M. Garnache is attending Seminar III. 10 years, 7 months ago
-
Supreet K. Dhillon is attending Seminar II. 10 years, 7 months ago
-
Pavandeep Birk is attending Seminar III. 10 years, 7 months ago
-
Benjamin Heuberger wrote a new post on the site Emotion, Brain, & Behavior Laboratory 10 years, 7 months ago
My earliest memory is standing in my crib as a toddler in the early morning, dressed in a onesie, and starring ahead through the open door into my apartment’s hallway. For years, I have asked myself if this ever actually happened or if this memory was simply triggered by some dream or other event that lead me to believe it. While I will never know if I actually remember being a toddler in a crib, there is no doubt memories are imperfect and malleable, and false memories are no stranger to our minds. In this blog post I will first discuss the role our moods and emotions play in the formation of false memories and then delve into the neural basis of these false memories.
Throughout this class, we have discussed the dimensional approach to characterizing emotions along scales of valence and arousal. Much of the research that has looked at the interplay of emotions and false memories has examined emotions along scales of valence and arousal. Damme (2013) manipulated the valence and arousal of individuals’ moods and then tested their memory (both recognition and free recall) of presented images. Low-arousal moods were associated with higher rates of false recognition, regardless of the valence of the mood.

One possibility suggested by Damme (2013) that may explain these results is that previous research has demonstrated arousal improves item-specific memory whereas low-arousal induces an overgeneral retrieval style. In other words, when we are in a state of high arousal, we may be more attune to the fine details of stimuli and encode these more effectively than when we are in low arousal. These results are in line with research by Corson & Verrier (2007) that found that whether participants were in a neutral, positive, or negative mood, their formation of false memories was related only to the level of arousal of their mood, not its valence.
A study by Storbeck & Clore (2011) also examined how affect influence false memory formation. Happy and sad moods were induced either before or after learning words lists that were designed to elicit false memories. These researchers found that sad moods reduced false memories only when induced before learning (the words they were subsequently tested on). These results are in conflict with those found by Storbeck & Clore (2011). This may be explained by the fact that Storbeck & Clore (2011) didn’t specifically examine arousal levels during memory testing, so the differential effects of happy and sad moods may be the result of a higher arousal in the sad mood state and lower level of arousal in the happy mood state.
What is the neural basis of false memories? Researchers have found many similar brain regions brain regions activated by both true recognition and false recognition. In a study comparing participants’ true and false recognition of words (some of which had been previously presented in a series of lists and some of which had not), PET scan analysis found that both true and false recognition were associated with increased blood flow to the medial temporal lobe—particularly in the vicinity of the left parahippocampal gyrus—as compared to baseline (figure 1). (Schacter et al.,1996). In a similar paradigm involving
[caption id="attachment_420" align="alignright" width="300"]
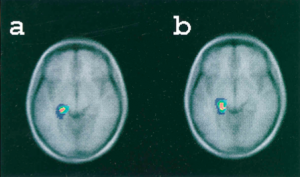 Figure 1: Blood flow increases in the left medial temporal lobe during both true recognition and false recognition. (Credit: Schacter et al. 1996)
Figure 1: Blood flow increases in the left medial temporal lobe during both true recognition and false recognition. (Credit: Schacter et al. 1996)exposure to images of colored shapes and a subsequent memory recognition test containing both old and new images under fMRI, Garoff-Eaton et al. (2006) found that increase in activity of the medial temporal lobe (right parahippocampal gyrus) and parietal cortex was seen in both true and false recognition. However, this similarity in activation with true recognition was only observed for false recognition of new shapes that were highly similar to the original shapes (related false recognition) and not for highly dissimilar new shapes (unrelated false recognition). In other words, false recognition of new dissimilar images did not show this same activation pattern as true recognition.
Yet while neural activation similarities exist, there also appear to be distinct regions of activity associated with each kind of recognition. In their study involving true and false recognition of previously-presented words, Schacter et al. (1996) found increased activation in the left tempoparietal cortex—particularly in the vicinity of the superior temporal cortex—during true recognition but not false recognition. False recognition of words was associated with greater increased activity in the orbitofrontal cortex, prefrontal cortex, and cerebellum as compared with true recognition. In another study, Baym & Gonsalves (2010) first exposed participants to a series of photographs while monitoring their neural activity with fMRI. They then exposed them to a misinformation phase (also under fMRI) that contained some true and some untrue sentences about the photos. After a time delay, participants’ memory recognition was tested. The researchers found that greater activation of the superior temporal gyrus during picture exposure predicted true memory compared with false memory, which is in line with the results of Schacter el al. (1996).
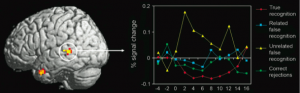
Figure 2: Increased activation of the left superior temporal gyrus during unrelated false recognition as compared to true recognition and related false recognition. (credit: Garoff-Eaton et al., 2006)
One hypothesis proposed by Schacter et al. (1996) to explain differential activation during false memory recall is that the greater activation of the tempoparietal region during true recognition as compared with illusory recognition may reflect the neural retrieval of auditory information of “true” words that were actually rehearsed and heard—information which would be available only in the case of true recognition and not in the case of false recognition (as these “false” words had never been presented or rehearsed previously). Garoff-Eaton et al. (2006) showed greater activation of left superior temporal cortex associated with unrelated false recognition as compared to true recognition (figure 2). They propose that unrelated false recognition could result from the application of a verbal label to a new shape on during the recognition phase that matched a verbal label they had mentally applied to one of the true shapes, which could explain activation of the left SPT, commonly associated with language processing.
As I have laid out here, mood—particularly in terms of valence and arousal—seems to modulate our formation of false memories. Furthermore, there appears to be compelling evidence for both similar and differential activation of brain regions during true recognition and false memory processes. However, while there is a large body of neural evidence that sheds light on the basis of false memories, research needs to be done that examines how exactly mood and emotion modulate the false memory formation on a neural level. Perhaps using similar fMRI or PET analysis discussed in this paper may help shed light on the seemingly competing claims of Damme (2013) and Storbeck & Clore (2011) by helping to quantify levels of arousal and valence in false memory experiments.
References:
Baym, C. L, Gonsalves, B. D. (2010). Comparison of neural activity that leads to true memories, false memories, and forgetting: An fMRI study of the misinformation effect. Cognitive, Affective, & Behavioral Neuroscience, 10, 339-348.
Corson, Y., and Verrier, N. (2007). Emotions and False Memories: Valence Or Arousal? Psychological Science 18, 208-211.
Damme, I. V. (2014). Mood and the DRM paradigm: An investigation of the effects of valence and arousal on false memory. The Quarterly Journal of Experimental Psychology, 66, 1060-1081.
Garoff-Eaton, R. J., Slotnick, S. D., Schacter, D. L. (2006). Not all false memories are created equal: the neural basis of false recognition. Cerebral Cortex, 16, 1645-1652.
Schacter, D. L., Reiman, E., Curran, T., Sheng Yun, L., Bandy, D., McDermott, K. B., Roediger, H. L. (1996). Neuroanatomical Correlates of Veridical and Illusory Recognition Memory: Evidence from Positron Emission Tomography. Neuron, 17, 267-274.
Storbeck, J., Clore, G. L. (2011). Affect Influences False Memories at Encoding: Evidence from Recognition Data. Emotion, 11, 981-989.
-
Katharina S. Fung is attending Seminar IV. 10 years, 7 months ago
-
jsanch08 is attending Seminar IV. 10 years, 7 months ago
-
Justin Cardarelli is attending Seminar IV. 10 years, 7 months ago
-
Emma Zimmerman is attending Seminar IV. 10 years, 7 months ago
-
Kevin Q. Nguyen is attending Seminar III. 10 years, 7 months ago
-
Supreet K. Dhillon is attending Seminar V. 10 years, 7 months ago
- Load More




