Drs. Suzanne Cunningham and John Rush
Revised from original syllabus by Dr. James N. Ross Jr.
OBJECTIVES
- Be able to recognize clinical signs and historical findings typical of cardiac disease and be aware of interspecies differences in these findings.
- Know the significance of mucous membrane pallor, injection, cyanosis, CRT, and hydration status.
- Be familiar with normal findings for jugular vein observation, mechanism of hepatojugular reflux, and significance of abnormalities of jugular distention or pulsation.
- Be aware of techniques of precordial palpation to assess ventricular enlargement; ventricular function; heart sounds and murmurs (thrills); rate and rhythm.
- Be familiar with the significance of normal and abnormal findings associated with arterial pulse assessment: rate, rhythm, character (hypo – hyperkinetic, rate of rise, rate of fall, pulse pressure).
- Know your cardiac auscultation! – Techniques, areas, murmur characterization (systolic vs. diastolic, point of maximal intensity, grade, “systolic ejection” vs. “holosystolic” murmurs), origin of heart sounds, heart sound intensity, splitting of heart sounds, gallops, detection of arrhythmias, etc.
INTRODUCTION
Proper management of the patient with cardiac disease necessitates a complete cardiovascular diagnosis. The development of a rational plan of management is based on a knowledge of:
- The underlying etiology (is the disease congenital, infectious, hypertensive, etc. in origin)
- The anatomical abnormalities present (which chambers are enlarged, which valves are affected, is there pericardial involvement)
- The physiological disturbances present (is there an arrhythmia or evidence of congestive heart failure or of myocardial dysfunction)
- The extent of the animal’s functional disability (how much and what type of activity is required to elicit clinical signs, what is the quality of the animal’s life in its environment).
Not infrequently, several different methods of examination are required to establish a diagnosis. They include the history, physical examination, electrocardiogram, thoracic radiographs, echocardiography, assessment of cardiac biomarkers, and, occasionally, specialized invasive procedures such as cardiac catheterization, angiocardiography, or myocardial biopsy.
HISTORY AND CHIEF COMPLAINTS
Animals with cardiac disease are usually brought to the veterinarian for examination because the owner perceives some problem. You will learn to ask questions and elicit specific information from animal owners to aid in the diagnosis and therapy – no small trick! You can increase the value of historical information from the owner if you are careful (tricky?) about the way you ask the questions.
Historical information relating to cardiac disease often relates to the degree of circulatory compromise of the animal. If the left heart is dysfunctional, fluid may accumulate in the lungs (pulmonary edema) resulting in cough, difficulty breathing, inefficient gas exchange, hypoxemia, and death. Right-sided heart failure results in congestion of the liver (with subsequent hepatic damage and necrosis), and accumulation of fluid in the abdomen (ascites), thorax (pleural effusion), or pericardial space. Animal owner complaints revolve around their perceptions of these and other abnormalities and can include cough or difficulty breathing, fainting or perceived seizure, or limited ability for exercise.
- Any of the following signs, or chief complaints, should prompt investigation for cardiovascular disease:
- Weakness, lethargy or fatigueReduced exercise tolerance
- Dyspnea, tachypnea, panting, coughing, orthopnea (respiratory distress)
- Syncope
- Cyanosis
- Abdominal distention
- Peripheral edema
- Weight loss, cachexia
- Hemoptysis
- Irregular heartbeat
- Weakness, fatigue, lethargy and reduced exercise tolerance are nonspecific and common to many diseases. Cardiovascular disease with reduced cardiac output and peripheral perfusion is but one cause, but reduced exercise tolerance is a common sign in many forms of heart disease. In an animal that exercises frequently, or is called upon to do work, a reduction in exercise tolerance is frequently the first clue to the presence of heart disease. However, as many of our animals rarely exercise (i.e. are either cats or couch potatoes), a reduction in exercise tolerance may not be noted until the animal is either symptomatic “at rest” or cannot perform the small bit of activity it is required to do (walk to the food bowl).
- Respiratory signs occur frequently in animals with cardiovascular disease but are also noted in animals with primary diseases of the airways and pulmonary parenchyma. The cough reflex may result from inflammatory, mechanical, chemical or thermal stimulation of the cough receptors. The history and physical exam can generally determine whether the cough is of upper or lower airway origin, helping to prioritize the likelihood that the cough results from a cardiac etiology. Recall, however, that cardiac and respiratory disease may occur together, and one may exacerbate the other. Paroxysms of coughing may precipitate syncope (so-called tussive syncope). Therapy of cough depends upon determining its precise cause and initiating specific therapy for the underlying disease. Symptomatic therapy should be considered only when the cause is idiopathic and/or it is useful to stop the prolonged self-perpetuating cycle of coughing.
- Dyspnea, i.e., difficulty breathing, is a cardinal sign of diseases affecting the cardiorespiratory system. Dyspnea may be associated with congestive heart failure (CHF), pulmonary thromboembolism, heartworm disease, spontaneous pneumothorax, non-cardiogenic pulmonary edema, or a variety of other causes. Nocturnal dyspnea, or difficulty breathing mostly at night, is a possible characteristic of left-sided congestive heart failure (CHF). Orthopnea (dyspnea when lying down) frequently occurs in CHF but may also occur in some patients with asthma and/or chronic obstructive airway disease. It is helpful to determine whether the animal is actually working hard to breathe (as opposed to panting) — if so, dyspnea always requires immediate further evaluation and therapy! Cardiac dyspnea usually begins as difficulty in breathing with exertion, progressing over time until the patient is dyspneic at rest. Again, a complaint of nocturnal dyspnea and restlessness (breathing with difficulty in the recumbent position at night, then getting up to pace around which results in some relief) is frequently associated with heart disease. Elevation of the diaphragm in the recumbent position (example – with marked ascites) also reduces lung volume and may contribute to dyspnea. Nocturnal dyspnea has also been termed “cardiac asthma”.
Recall the pathogenesis of pulmonary edema with heart disease — an increase in left atrial and pulmonary venous pressures results initially in engorgement of the pulmonary vasculature and an increase in pulmonary capillary pressure. Initially, the edema is interstitial, and the lungs become less compliant, the resistance of small airways increases, and there is an increase in lymphatic drainage in attempt to maintain a constant extravascular fluid volume in the lung. This usually results in tachypnea, and tachypnea appears to be compensatory in the sense that it increases lymphatic flow by augmenting ventilatory pumping of lymphatic vessels. At this stage, there are no auscultatory or radiographic signs of pulmonary edema (!), though pulmonary venous dilation may be apparent radiographically. As elevations in pulmonary venous pressure increase in severity, the “safety mechanisms” of the lung that serve to keep the accumulation of extravascular fluid in check are overwhelmed. This results in a net accumulation of fluid in the interstitial spaces of the lung, with accompanying signs of worsening tachypnea, cough and the development of radiographic signs of pulmonary edema (an interstitial pulmonary pattern). Note that the edema is still purely interstitial at this stage. With further elevations in intravascular pressure, however, there is destruction of the tighter junctions between alveolar lining cells and flooding of the alveoli, resulting in progressive dyspnea and radiographic evidence of an alveolar pattern in the lungs.
- Cyanosis, the bluish color of the skin and mucous membranes, results from an increased amount of reduced hemoglobin or hemoglobin derivatives in the small blood vessels. It may be brought about either by an increase in the quantity of venous blood in the skin (as a result of dilatation of the venules and venous ends of capillaries) or by a reduction in the oxygen saturation of the capillary blood. It is the absolute rather than the relative amount of reduced hemoglobin (need ≥ 5 gm/dl) which is important in producing cyanosis. Note that cyanosis is an insensitive indicator of hypoxemia and arterial oxygen saturation is typically reduced to less than 85-90% before central cyanosis becomes apparent!
- Central cyanosis causes both mucous membranes and skin to be affected and is often due to central shunting of blood, severe respiratory disease, poor gas exchange in the lungs, or a reduction in inspired FiO2.
- Peripheral cyanosis may be caused by vasoconstriction and diminished peripheral blood flow as may occur in shock and low-output heart failure. Polycythemia is particularly striking in patients with cyanotic congenital heart disease and may also contribute to impaired peripheral perfusion. Peripheral cyanosis can also be appreciated secondary to obstruction to blood flow from thrombus formation (e.g. cats with arterial thromboembolism).
- Differential cyanosis, in which the caudal mucous membranes are cyanotic but the cranial mucous membranes are pink, is virtually pathognomic for a right-to-left shunting patent ductus arteriosus (PDA). Exercise may be needed to precipitate this finding.
- Right-sided or biventricular congestive heart failure (CHF) may result in hepatomegaly, ascites, hydrothorax (pleural effusion) or, peripheral edema. The presence of ascites is characterized by abdominal distension and a ballotable fluid wave. Large pleural effusions result in dyspnea and diminished lung sounds in the ventral lung fields (ventral fluid line) Since edema, ascites and pleural effusion can be caused by many things other than CHF, other evidence of heart disease (e.g., cardiac enlargement, jugular venous distension, a cardiac gallop, etc.) provides an indication that the edema/effusion is cardiogenic in origin.
- Syncope is defined as a sudden and transient loss of consciousness due to transient interruption of blood flow to the brain. It may result from poor cerebral circulation, a result of a transient decrease in cardiac output and/or blood pressure, or from a shortage of energy substrates delivered to the brain through blood flow. Episodes of severity less than syncope, i.e. where a degree of consciousness is maintained, may be classified as “collapse” or “weakness”; the latter may be described as “pre-syncope”. Cardiogenic syncope in small animals is usually an acute, transient attack progressing from generalized muscle weakness through ataxia to collapse in a short period of time with a brief period of loss of consciousness. The patient during the latter may be motionless with relaxed muscles, or may display uncoordinated muscular activity or jerks giving the impression of seizure-like activity. Muscle sphincter control can be lost resulting in involuntary urination and/or less frequently defecation, and the animal may cry out during the episode. Recovery is usually rapid with the episode lasting only a few seconds and usually recovery in a few minutes. This rapid post-syncopal recovery is one of the key differences between syncopal events and seizures, with seizure activity typically being followed by a post-ictal phase of variable duration during which the animal displays behavioral changes or is not mentally appropriate.
- Once the chief complaint or signs are noted, take time to develop
the history by
quizzing the owner with questions and probing for clues with attention
to the following:
- When sign(s) began, their frequency, duration, timing, associations.
- Specific attention to exercise and activity, how much.
- Respiratory signs, cardiac cough: low pitched, resonant, paroxysms on rising, described as gagging, productive, nocturnal
- Diet, dietary supplements
- Environment
- Prior history, treatment, drugs, efficacy of prior therapies, etc.
- Syncope, gait, lameness associations
- Patients with cardiovascular disease may also be asymptomatic. It is becoming more and more common to detect cardiac disease as a result of some abnormal physical finding such as a heart murmur, enlarged cardiac silhouette on the thoracic radiograph, increased cardiac biomarker (e.g., NT-proBNP or cardiac troponin I), or abnormal ECG findings detected during the “well-animal” examination or when examining an animal for some other primary complaint.
GENERAL INSPECTION
Observe the animal’s general body condition, temperament, attitude and ability to ambulate. Observations that may suggest the presence of heart disease include weakness, loss of epaxial muscle mass (cardiac cachexia), abdominal distension, abducted elbows, dyspnea, hyperpnea or other abnormalities in respiratory rate, effort, or regularity. Be sure to observe the animal’s respiratory character from “afar” before you begin the physical exam! The mucous membranes of the eyes and oral cavities may be compared to caudal mucous membranes if looking for differential cyanosis. Cyanosis, icterus, petechia, injection or paleness may give clues to the underlying problems (e.g., hypoxemia, right- to-left shunt, hepatobiliary disease, hemolysis, thrombocytopenia, polycythemia, or anemia). The vessels of the ocular fundus may give clues to the presence of systemic hypertension. Checking capillary refill time is a crude estimate of cardiac performance. One area of the examination by inspection that has been relatively neglected is the careful observation of the jugular veins.
OBSERVING THE JUGULAR VEINS
- Examination of the jugular veins gives an indication of central venous pressure as well as valuable information about right-side cardiac events. The jugular veins directly reflect right ventricular filling pressure during diastole, and right atrial pressure during systole. The jugular veins can be adequately examined with the animal standing, sitting or in sternal recumbency; standing or sternal recumbency is preferred. The jugular vein will not reflect right atrial pressure when the animal is lying down. Elevating the animal’s muzzle and turning the head slightly to the left facilitates inspection of the right jugular vein (and vice versa). While a thick hair coat can sometimes obscure jugular venous distension, the hairs often move with jugular pulsations and facilitate the identification of the various pulse waves.
- Central venous pressure is estimated by determining the height the jugular vein is distended above right atrial level. By occluding the vein at the thoracic inlet, it easily can be identified as it becomes distended through filling from above. Once identified, the pressure at the thoracic inlet is removed while continuing to observe the vein. If the jugular veins are collapsed (below the thoracic inlet), the central venous pressure is subnormal, and blood volume is frequently inadequate. On the other hand, the estimated height of venous distension above the level of the right atrium is a relatively accurate reflection of central venous pressure (1 to 5 cm is within the normal range). Most commonly, elevated venous pressures are due to elevated right ventricular end- diastolic pressure (i.e., right ventricular failure). The jugular veins also are markedly distended in cardiac tamponade and other pericardial disease, and in animals with iatrogenic fluid overload. In most cases, if the jugular vein is observed in the bottom 1/3 of the neck then the right atrial pressure is probably OK (maybe a tiny bit higher in some cats). If the jugular vein is pulsating in the middle 1/3 of the neck then the CVP is elevated. And if the jugular vein extends all the way to the top 1/3 of the neck (close to the angle of the mandible) then the CVP is markedly elevated and in many cases right-sided CHF will be present.
- In addition to estimating central venous pressure, the jugular venous pulse waves may yield clinical “pearls” in diagnosis. A positive a wave occurs with right atrial contraction and thus follows the P wave of the electrocardiogram but precedes ventricular contraction and the upstroke of the carotid pulse. In the normal jugular venous pulse, the a wave is followed by the x descent as the right atrium relaxes and right atrial pressure falls further during early right ventricular systole. The next positive wave, which is often imperceptible, is termed a c wave, which reflects the increase in atrial pressure with the upward bulging of the tricuspid valve as it closes during isovolumic systole. After the C wave, the v wave is a second major positive wave in the jugular venous pulse. It occurs in late systole and ends in early diastole. After the v wave, as the tricuspid valve opens, venous pressure falls, and a second negative wave, the y descent, occurs in early diastole.
- Right ventricular failure, cardiac preload, respiration, and cardiac arrhythmias can all influence the jugular vein observation. Inspiration results in a lowering of intrathoracic and right atrial pressure, and because the right side of the heart tends to fill more with inspiration, there is an exaggeration of the x and y descents with inspiration.
- The a wave in the jugular vein requires atrial contraction, and the a wave would be absent during atrial fibrillation or atrial standstill.
- Giant a waves or very large a waves are associated with decreased right ventricular compliance as may be seen in marked right ventricular hypertrophy from causes such as pulmonic stenosis, and tricuspid stenosis could also result in giant a waves.
- Cannon a waves can occur when the right atrium contracts against a closed tricuspid valve, as can occur with third degree AV block, or ventricular premature beats or ventricular tachycardia.
- Assessment of hepatojugular reflux should also be performed while observing the jugular veins. In this technique, the cranial abdomen is compressed while observing the jugulars. Abdominal compression increases venous return and tends to force blood out of a congested liver. This test is useful whenever the jugular venous pulse is borderline elevated or when latent right ventricular failure or minimal tricuspid regurgitation is suspected. Abdominal compression forces blood into the thorax and a failing or dilated right ventricle may not be able to accept the augmented venous return; therefore, a rise in mean venous pressure is detected. Normally, abdominal compression will not elevate the venous pressure very much and, if so, the elevation is not sustained. In CHF or tricuspid regurgitation, however, the maneuver results in an elevation in jugular venous pressure, and it persists throughout the time the positive pressure is applied to the abdomen. This is referred to as a positive hepatojugular reflux. It is often helpful to auscultate using the bell at the left ventricular apex while performing this test, as it sometimes brings out a gallop rhythm which was not apparent at rest.
PALPATION
- Precordial palpation is part of the cardiovascular examination, and this refers to palpation of the thorax over the regions the heart and ventral thorax (precordium). In normal individuals, cardiac motion is represented by an apex beat or apex impulse which is appreciated in a small roughly 2 cm area, usually at or around the left fifth intercostal space, slightly below the costochondral junction. Right ventricular activity may be felt to a lesser degree on the right side of the chest in approximately the tricuspid area of auscultation (in the fourth intercostal space). For most dogs and cats, precordial palpation can be best accomplished by placing both hands on the chest with the fingers pointing ventrally toward the sternum. For many people, the palm of the hand or the fleshy inner part of the proximal phalanx (not the fingertips) is the most sensitive region for palpation of cardiac thrills. Depending upon the animal’s size, the fingers are spread to fit the intercostal spaces so that the third to the seventh intercostal spaces are appreciated.
- Cardiac hypertrophy and dilation create abnormal systolic and diastolic events which may be detected on palpation. Ideally, precordial palpation should precede auscultation of the heart, as palpation may help direct your attention to certain regions during auscultation. Normally, the left ventricular apical impulse is felt as an early outward thrust during systole and is typically located in the left fifth intercostal space. In conditions resulting in a volume overload of the left ventricle (mitral regurgitation or aortic insufficiency), the apical impulse may be hyperkinetic or appreciated as an increase in the amplitude of the impulse. As the heart dilates, the left ventricular volume increases, resulting in a more posterior displacement of the apical impulse (to the 6th interspace or further back) as well as the increase in the size of the actual contact area of the apex beat. With a pressure overload of the left ventricle, the intensity of the LV apical impulse can increase, and this is sometimes described as a left ventricular “heave” or “thrust.” A diminished apex beat may be appreciated in conditions of shock, severe hypovolemia, or severely depressed left ventricular systolic dysfunction. Palpation of a vigorous right ventricular impulse suggests right ventricular enlargement and, as the right ventricle enlarges, the normal brief thrust becomes more sustained on the right side of the chest. Other differentials that must be considered when abnormalities are detected on precordial palpation are masses or effusions or diaphragmatic hernia displacing the heart.
- A large left ventricular end-diastolic volume as well as a significant depression of left ventricular function may be associated with the presence of an audible S3 (i.e., an S3 gallop) which, if intense enough, may also be palpable at the left apex. Typically, these animals have left ventricular failure and a decreased ejection fraction. A fourth heart sound also may be palpable and is related to decreased left ventricular compliance, generally a result of concentric hypertrophy.
- Loud murmurs are perceived as “thrills” on the chest wall. These palpable murmurs correlate with an intensity of Grade V/VI or greater. These thrills can be localized to the cardiac apex in the case of mitral regurgitation, to the cardiac base in the case of pulmonic stenosis or patent ductus arteriosus, and to the right side of the chest with ventricular septal defects.
- Studies have shown that the palm of the hand and the proximal metacarpals are most useful for the initial localization of palpable cardiac motion and the detection of precordial thrills, whereas the fingertips are best for precise localization and assessment of left and right ventricular activity.
ARTERIAL PULSES
- When the pulse is palpated in any artery, it is the changing arterial dimensions due to the pulse pressure, or the difference between the systolic and diastolic pressure in the artery that is perceived. The absolute level of pressure cannot be ascertained without measuring it. Indirect measurement of blood pressure is feasible and should be performed routinely in dogs and cats. But some things can be perceived with palpation can supplement information about the animal even if the blood pressure has already been measured.
- While the femoral artery is the easiest artery in which to obtain a pulse in most small animals, pulses can be compared in various arteries including the femoral artery, cranial tibial artery, carotid artery and under anesthesia the tongue artery. The usual technique for palpating the pulse is to compress the artery with the forefinger until the maximum pulse is sensed. It may be necessary to apply varying degrees of pressure to appreciate all aspects of the pulse. One attempts to assess the sharpness of the upstroke, the systolic peak of the pulse and the diastolic slope of the pulse as it falls.
- The pulse pressure is determined principally by the left ventricular stroke volume; though the compliance, capacity and runoff from the arterial system with each heartbeat certainly contributes. An increase in pulse pressure is perceived as a bounding, or hyperkinetic arterial pulse and generally is due to an increased stroke volume and/or an increased diastolic “runoff” as in patent ductus arteriosus with a left-right shunt, aortic insufficiency, or peripheral arteriovenous fistula.
- A small, weakened pulse is called pulsus parvus. Pulsus parvus is present in conditions with a diminished left ventricular stroke volume and a narrow pulse pressure. Differentials include hypovolemia, left ventricular failure, pericardial disease, and aortic stenosis. In aortic stenosis the systolic peak is not only diminished in intensity, but also delayed and may referred to as pulsus parvus et tardus. In aortic stenosis, a thrill on the chest wall is often felt and a harsh systolic ejection murmur is heard in the aortic valve area on auscultation.
- Pulsus alternans is characteristic of severe left ventricular dysfunction and heart failure. Pulsus alternans is characterized by alternating pulse pressure amplitude despite a regular cardiac rhythm. Commonly, such animals also have an S3 gallop. Pulsus alternans must be differentiated from pulsus bigeminus where there also is a regular alteration in pulse pressure that is caused by a premature contraction following each regular heartbeat.
- Pulsus paradoxus is a helpful finding in the dog, as it is almost always associated with pericardial effusion and cardiac tamponade. Pulsus paradoxus is an accentuation of the pulse on expiration and a reduction in the amplitude of the arterial pulse during inspiration. It is not unusual for the dog with cardiac tamponade to have rather weak pulses which disappear entirely during inspiration. Pulsus paradoxus also has been associated with airway obstruction and superior vena caval occlusion in man.
- The arterial pulse character is subject to beat-to-beat variations in cardiac filling (preload) due to the variable diastolic interval. This may be readily evident if there is sinus arrhythmia but is dramatic when there is a significant arrhythmia such as atrial fibrillation, premature atrial or ventricular beats, or tachyarrhythmias (atrial or ventricular). In the setting of these arrhythmias, diastolic filling may be so reduced that no pulse is generated with the ventricular contraction. This results in an S1 sound, a diminished or absent S2 sound, and is associated with a diminished or absent arterial pulse. Intraventricular pressure recordings show that the pressure generated in the left ventricle may be insufficient to open the aortic valve at all, or that the pressure generated may cause only a diminutive aortic pressure pulse in association with a greatly diminished ejection volume. The lack of pulse generated by ventricular contraction is termed a pulse deficit; i.e. it is entirely possible for there to be a greater number of cardiac contractions (noted by auscultation) than pulses generated and palpated by the fingers. For this reason, we make a clinical distinction between the heart rate and the pulse rate. Another term used to signify the same phenomenon is asynchronous pulses, i.e., indicating that the pulses are not synchronized with the cardiac contractions.
AUSCULTATION
- Understanding normal and abnormal physical exam findings is intimately tied to a thorough understanding of the cardiac cycle, including the sequence and timing of events, pressures within the chambers, and interrelationships between pressures, blood flow, blood volume, and other factors.
- All sound is the result of vibrations propagated through a medium such as air.
- How well you can hear a sound using your stethoscope depends on its intensity (e.g., decibels) and whether the frequency of sound (pitch) is within a range that your ears are sensitive to. Much of the sound energy produced by the cardiovascular system is NOT within a frequency range that your ears are adapted to hear well.
- The stethoscope itself is part of the acoustic transmission system during the physical examination. The stethoscope can be used to accentuate specific frequencies (low versus high pitch). Most stethoscopes come with both a bell and a diaphragm, and using both the bell and the diaphragm give you the advantage of all the available tricks to hear murmurs, gallops and heart sounds.
- Sounds from specific cardiac pathology are often conveyed to a characteristic location on the thorax. Sounds emanating from the mitral valve (murmurs, S1, clicks), for example, typically are loudest at the left apex of the heart, near the 5th or 6th intercostal space.
- Sound intensity and character also relates to the medium of transmission. Increased body mass (muscle, adipose) between the sound source and stethoscope increases the attenuation; loss of body mass has the opposite effect. For this reason, heart sounds may seem “muffled” in large or obese animals and accentuated in small or cachectic patients.
- The heart sounds are NOT the result of valve leaflets “hitting” each other; these structures are wispy thin and nearly massless. Sounds are the result of vibrations brought about by rapid deceleration of moving masses of blood, e.g. when a valve closes.

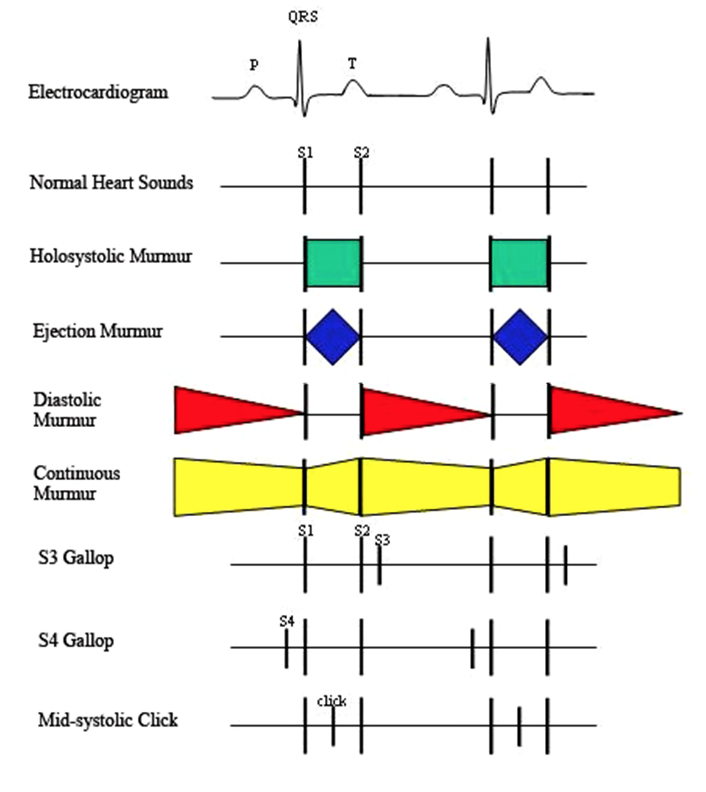
- The most obvious sounds apparent on auscultation of the normal heart are the S1 and S2 sounds associated with closure of the AV valves (mitral and tricuspid) and semilunar valves (aortic and pulmonic), respectively. Consequent to ventricular activation (shortly after the start of the QRS complex), the ventricles begin to contract, and the pressure starts to rise within the ventricle. As ventricular pressure exceeds atrial pressure, the AV valves close and the heart and blood oscillates abruptly, resulting in the S1 sound. This sound may be audibly split (split S1) in normal dogs and in large animals due to slightly asynchronous closure of the AV valves. A split S1 can also be a clue to a pathological condition that accentuates the asynchrony of AV closure, e.g., bundle branch block or premature ventricular beat where activation of one ventricle is delayed relative to the other.
- The intensity of S1 is influenced by hemodynamic conditions as it relates to the vigor of the initial contraction of the heart. Hence it is accentuated with increased preload or contractility; its intensity decreases with increasing heart rate. A diminished S1 is a clue to the diagnosis of hypovolemic shock, cardiac tamponade, dilated cardiomyopathy, and other conditions that decrease preload or contractility. S1 intensity is increased with hyperdynamic conditions (fear, exercise and excitement, mitral valve insufficiency, anemia, fever). We expect the mitral component of S1 to be more intense than the tricuspid component due to the greater vigor of left ventricular contraction.
- The S2 sound is brought about by closure of the semilunar valves; blood literally begins to flow retrograde in the aorta and pulmonary arteries and is brought to rest (decelerated) abruptly by valve closure. This sound normally has a higher pitch than the S1 sound, contributing to a “crispier” character; we imitate the S1 with a longer, lower pitched “lub” syllable, the S2 with the higher, briefer “dup”. The S2 sound is loudest over the aortic (and pulmonic) valve areas, i.e., the heart base.
- In normal larger animals (e.g., humans, horses, and cattle), we can often hear splitting of the S2 sound due to asynchronous closure of the semi lunar valves. Right ventricular ejection normally takes a little longer than left ventricular; the pulmonic component of the S2 sound occurs later than the aortic component. Furthermore, the thoracic motion of inspiration literally draws blood into the thorax, preferentially into the right ventricle due to its greater compliance, and right ventricular ejection takes even longer so that splitting of the S2 sound is accentuated (more apparent) with inspiration. Respiratory accentuation of the split (or splitting that can only be appreciated on inspiration) is referred to a physiological split (of the S2 sound).
- S2 splitting can be accentuated by conditions that prolong or abbreviate ejection of either ventricle relative to the other. Delayed right ventricular (RV) ejection from increased RV afterload (e.g., due to pulmonary hypertension) is expected to result in further delay of P2 after A2. The increased pressures associated with pulmonic valve closure also tend to increase the intensity of the sound; it becomes both louder and split. Delayed closure of the aortic valve due to increased left ventricular afterload (systemic hypertension, aortic stenosis) can result in paradoxical splitting (or “reverse” splitting) of the S2 sound where A2 is delayed beyond P2. The paradoxical aspect is that the splitting diminishes with inspiration; inspiration prolongs right ventricular ejection as before, but this results in the P2 sound getting closer to A2, unlike the physiological split.
- Gallops sounds are abnormal diastolic sounds. An S3 gallop relates to a sudden decrease in the inflow velocity of blood entering the ventricle during early diastole, i.e., a rapid deceleration of blood flow. An S4 gallop occurs with atrial contraction, once again resulting from the deceleration of blood flow into a typically stiff ventricle, but the inflow is induced by atrial contraction in late diastole. Gallops sometimes can be accentuated by increasing venous return during auscultation with sustained pressure on the cranial portion of the abdomen.
*The presence of an S4 gallop suggests ventricular hypertrophy and reduced ventricular compliance. The presence of an S3 gallop suggests a large dilated failing ventricle.*
- Accurate timing of the sound or murmur may require relating it to another event in the cardiac cycle such as the arterial pulse, the jugular venous pressure waves, or the apical impulse. Heart rate may be slowed by carotid or ocular pressure in animals with some tachyarrhythmias. Once a sound (or murmur) is identified, one should observe alterations in its timing or intensity during the various phases of respiration. In general, sounds and murmurs originating from the right side of the heart tend to increase during inspiration, whereas sounds originating from the left side tend to increase during expiration.
- Cardiac arrhythmias are usually first and best appreciated on auscultation. Identification of a cardiac arrhythmia on physical examination subsequently requires an electrocardiogram to determine the exact nature of the arrhythmia.
- Each of the valve areas (mitral, aortic, pulmonic and tricuspid) should be auscultated using the “inching” technique with the stethoscope inched along the thoracic wall to the respective areas.
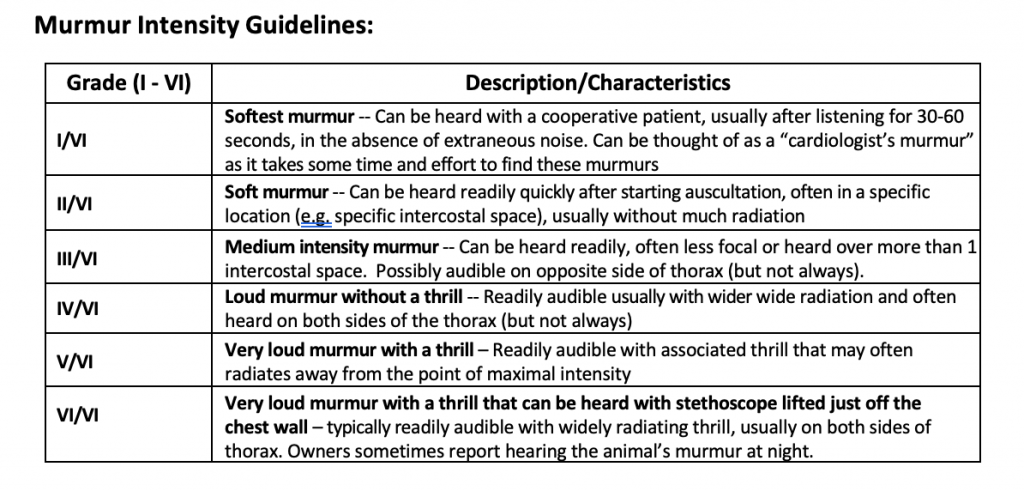
- Abnormal heart sounds and murmurs should be characterized by their period in the cardiac cycle, by their intensity, by their location on the chest wall where maximal intensity occurs (point of maximal intensity, or PMI) and by their quality. Murmurs are graded in loudness from a Grade I, which is the softest that can be heard, to a Grade VI which is audible with the stethoscope removed from contact with the chest. Systolic murmurs are further classified as to whether they last throughout systole (holosystolic, synonymous with pansystolic) or whether they occur principally during midsystole with a crescendo/decrescendo nature, in which case they may be termed systolic ejection murmurs.
- Holosystolic (pansystolic) murmurs tend to mask both heart sounds since there is flow between two chambers which have widely different pressures during systole. These murmurs include mitral insufficiency, tricuspid insufficiency, and ventricular septal defects. The latter two are heard with maximal intensity on the right side of the chest, whereas mitral insufficiency is heard with maximal intensity at the left ventricular apex.
- Systolic ejection murmurs may result from pulmonic stenosis (heard with maximal intensity in the pulmonary area), aortic stenosis (heard with maximal intensity in either the aortic area on the left side of the chest or the heart base on the right side of the chest), aortic flow murmurs, tetralogy of Fallot (similar to pulmonic stenosis), and atrial septal defect (similar to pulmonic stenosis).
- Diastolic murmurs are most commonly due to aortic regurgitation. Generally, they are decrescendo, being loudest after the second heart sound when aortic pressure is highest. Pulmonary insufficiency also can cause a diastolic murmur with marked insufficiency or concurrent pulmonary hypertension, as can mitral or tricuspid valve stenosis, but these are rare entities in domestic animals.
- Continuous murmurs occur throughout systole and diastoleand are associated with systemic arterio-venous fistulas such as patent ductus arteriosus (PDA). In a PDA, elevated pulmonary pressure can cause the murmur to fade out in late diastole.