Faculty Syllabus: Acquired Myocardial Disease
ACQUIRED MYOCARDIAL DISEASE
John E. Rush, DVM, MS, DACVIM (Cardiology), DACVECC
Cummings School of Veterinary Medicine At Tufts University
Last Updated 03/26/2020
OBJECTIVES
1. Define myocarditis
2. List 3 possible clinical presentations or sequela of myocarditis
3. Be familiar with the diseases in italics in the syllabus, especially the following disease processes which result in myocardial disease.
a. Parvovirus
b. Borrelia burgdorferi
c. Toxoplasmosis
d. Tripanosomiasis
e. Thyroid disorders
f. Doxorubicin-induced cardiomyopathy
g. Electrical injury
h. Vitamin E and Selenium deficiency
i. Catecholamine cardiotoxicity
j. Hemorrhagic shock
k. Hypertensive heart disease
DISEASES OF THE MYOCARDIUM
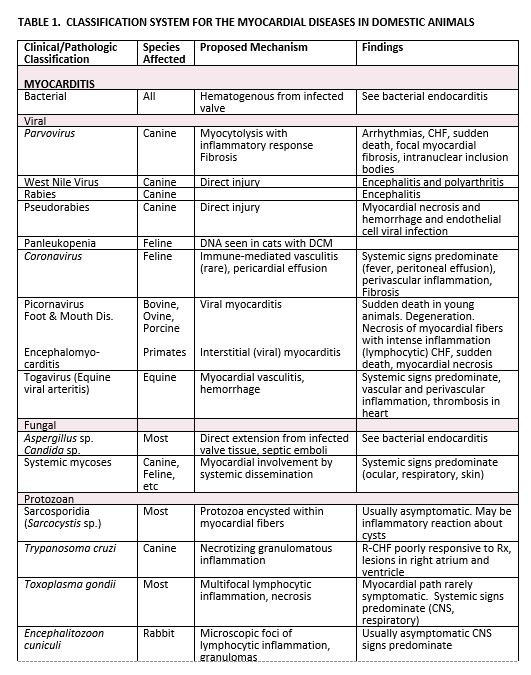
Myocardial diseases are characterized by processes or disorders in which heart muscle involvement is the predominant cardiac lesion. The endocardium and epicardium may also be affected by simultaneous involvement or extension of the myocardial process. Coronary artery disease is a much less common consideration in domestic animals than in man, but when coronary artery thrombosis occurs then myocardial dysfunction is often a result. Myocardial diseases of unknown cause or association are classified as primary cardiomyopathies, while those of known cause or association are classified as secondary myocardial diseases. With these basic objectives in mind a classification system for the myocardial diseases recognized in the domestic animals is presented (Table 1).
Myocarditis: The term myocarditis indicates an inflammation of the heart muscle. While this may be the result of primary muscle disorders and certain immune-mediated disease processes, most commonly it occurs as a sequela to some infectious agent. Many bacterial, viral, rickettsial, fungal and parasitic diseases have been shown capable of causing myocarditis, either in the natural host or by experimental infection of a laboratory animal. In the natural infection, clinical signs may be a direct result of myocardial involvement and the consequences of this that ensue, but more frequently systemic signs predominant the clinical picture and the myocarditis unrecognized until it is well developed, or post mortem examination is carried out. Histopathologic findings in myocarditis include inflammation and edema, especially inflammation with lymphocytes and macrophages. There is evidence of myocardial damage and necrosis leading to myocyte loss, which accounts for the clinical findings of myocardial failure. Fibrosis and fatty replacement may also be noted.
Myocardial involvement by these transmissible diseases varies considerably. When mild, cardiac findings may be totally normal to clinical examination or may be obscured by other localized organ involvement or multisystemic signs. The earliest recognizable findings are sinus tachycardia, cardiac arrhythmias and non-specific ST-T changes in the electrocardiogram. When myocardial involvement is more encompassing, cardiomegaly, tachy- and brady- arrhythmias, thromboembolism, congestive heart failure and sudden death are the expected events. Unrecognized or asymptomatic viral myocarditis has been postulated as a cause of idiopathic dilated cardiomyopathy in man.
Bacterial myocarditis usually occurs as a complication of bacterial endocarditis by either direct extension from infected valvular tissue or hematogenous spread. Many pathogenic bacteria have been incriminated. Immune-suppressed patients are at increased risk. This complication of bacterial endocarditis will be discussed under that subject in a separate lecture.
Viral myocarditis is an uncommonly recognized cause of myocardial disease in the domestic animals. In man over 22 viruses have been proven responsible for causing myocarditis.
In viral myocarditis the effects on the heart muscle are quite varied. In experimental animals, virus has been shown to replicate in cardiac tissue without clinical or light microscopic abnormalities. When microscopic lesions are present they may be focal or diffuse, and all portions of the heart may be affected including myocytes, interstitial tissue and even specialized tissues such as nodes and conduction pathways. Classically, viral myocarditis is an inflammatory lesion characterized by myocytolysis and necrosis with a predominantly mononuclear (usually lymphocytic) cell infiltration. In chronic, healed lesions myocardial fibrosis is the major finding.
It has been proposed that, in some cases, the myocarditis resulting from viral infections develops due to immune-mediated mechanisms, rather than direct injury of the myocytes by the virus. This has been based on: (1) myocyte necrosis begins after virus concentration in the heart has begun to decrease; (2) virus is not always detected in the myocardium at the time when cellular and humoral immune responses are maximal; (3) in some viral infections, viral replication in the heart is not a direct cause of the necrosis.
1. Parvovirus (dog)
a. Peracute form
-Puppies 3 to 8 weeks of age
-Typically acute CHF, sudden death; treatment is unrewarding
-Diffuse lymphocytic myocarditis
-Intranuclear basophilic inclusion bodies
-Incidence has decreased, likely related to widespread vaccination and maternal antibody protection
b. Delayed onset
-Puppies 3-5 months or older
-CHF, ventricular arrhythmias
-Dilated ventricles, scattered white foci over epicardium and endocardium
-Myocardial necrosis, fibrosis
-Postulated to be due to Parvo, although etiology is less well documented than form in puppies
2. Picornavirus
a. Foot and mouth disease (cattle, sheep, pigs, goats)
-Mortality rate approaches 50% in young animals
-Type C virus may cause myocarditis in adults
-Lymphocytic myocarditis with hyaline necrosis and scattered neutrophils
b. Encephalomyocarditis (pigs, primates, mice)
-Acute CHF – young pigs are particularly susceptible
Dilated hearts, scattered white streaks in right ventricle
Lymphocytic myocarditis with myocyte necrosis and calcification
Rats serve as the reservoir host of infection
3. Coronavirus (Cats)
a. Dilated cardiomyopathy-like syndrome in young kittens
b. Immune-mediated vasculitis in adult cats
-Non-cardiac, multisystemic signs usually dominate the clinical picture
-Pericardial effusion
-Myocardial involvement is rare
Fungal myocarditis is very rare in the domestic animal. In man, however, this is a well- recognized and dreaded problem. It may occur as a complication of the disseminated systemic mycoses (i.e. actinomycosis, blastomycosis, histoplasmosis, coccidioidomycosis); secondary to fungal endocarditis, typically Candida (e.g. most commonly seen following valve replacement surgery); and as a sequela to systemic moniliasis or aspergillosis in infants and in debilitated adults receiving prolonged antibiotic and/or immunosuppression therapy or with AIDS. Myocardial involvement in fungal myocarditis is usually characterized by the presence of granulomas or microabscesses. Only rarely are clinical manifestations of myocardial disease evident (see Table 1).
Spirochetal myocarditis. Lyme disease, Borrelia burgdorferi, has been reported to cause cardiac pathology in man and dogs. The organism seems to have a predisposition to affect the conduction system and variable degrees of AV block are common.
Protozoan infections are a common cause of myocardial lesions in the domestic animal, but are rarely responsible for the development of clinically significant myocarditis. When clinical signs are present, they are usually multisystem in origin or the result of non-cardiac, localized organ involvement. Myocardial effects, when present, are probably a combination of the pathogenic effects of the protozoan organism on the myocardium as well as the immune response generated by the affected animal.
1. Sarcosporidia, Sarcocystis sp (Aquatic Birds, Most Mammals – Particularly Herbivore)
a. The organisms result in cyst formation (i.e. sarcocysts) in cardiac and skeletal muscles throughout the body.
b. In most instances the cyst causes displacement of the sarcolemma without any inflammatory reaction and clinical signs are absent.
c. Overwhelming infection leading to clinical signs and death has been reported in calves.
2. Trypanosomiasis; Chagas’ Disease (Dog)
a. A serious and frequently fatal disease in Texas, Mexico, South and Central America caused by Trypanosoma cruzi.
b. Enzootic in wild animals in southern United States (armadillos, rodents)
c. Responsible vector – Reduviidae or “kissing” bugs (Triatoma sp).
d. Causes severe myocarditis affecting primarily the right atrium and ventricle, resulting in right-sided CHF.
e. Necrotizing granulomatous myocarditis associated with both intracellular and extracellular amastigotes of the organism.
3. Toxoplasmosis (Cat, Dog, Others)
a. An intestinal coccidian of cats – Toxoplasma gondii
b. Usually non-cardiac signs predominate owing to multisystem involvement (i.e. gastrointestinal, respiratory, CNS, ocular)
c. Cardiac lesions are found most commonly in the dog and cat, but are rarely responsible for clinical signs; scattered pale myocardial lesions may be seen grossly with microscopic findings of necrotizing myocarditis associated with scattered pseudocysts.
4. Encephalitozoonosis (Rabbits, Other Laboratory Rodents)
a. Caused by a microsporidium that is an obligate, intracellular protozoan parasite – Encephalitozoon cuniculi.
b. Urine-oral passage is the most important route of transmission in a rabbit colony, although fecal-oral, respiratory and transplacental transmission may also occur.
c. Most infections are chronic and subclinical, diagnosed at post mortem examination. CNS signs predominate when signs are present (i.e. torticollis, paresis, convulsions, and death). Myocarditis may develop and be responsible for clinical signs and sudden death in young rabbits.
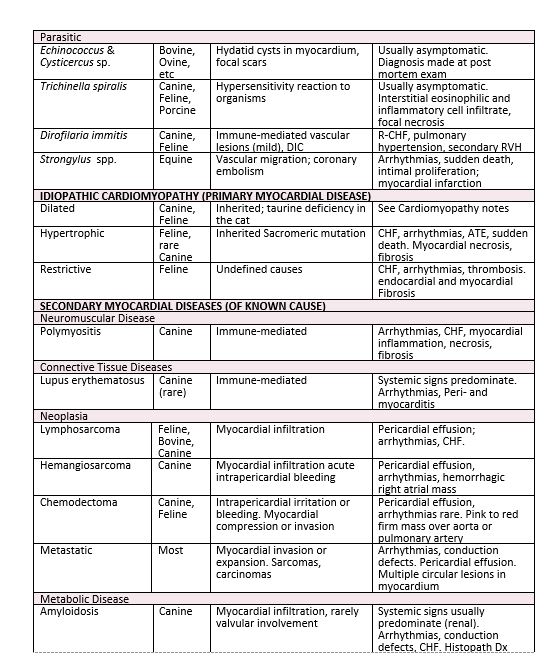
Myocardial lesions secondary to a variety of parasitic diseases have been reported in man and the domestic animal (see Table 1). These may be the result of a hypersensitivity or non-specific inflammatory response to the presence of larvae or larval migration (e.g. Trichinella spiralis, filariasis) or a reaction to the presence of encysted parasitic forms within the myocardium (e.g. echinococcosis, cysticercosis). Less commonly vascular lesions induced by the presence of larvae (e.g. dirofilariasis, strongyloidiasis) are responsible for the myocardial lesions. The myocardial changes caused by these parasitic diseases are usually mild and asymptomatic, being unrecognized until post mortem examination. Only Strongylus spp in the equine and Trichinella spiralis infections in man impose a threat to myocardial performance and the potential for congestive heart failure and sudden death.
Secondary Myocardial Disease
The secondary myocardial diseases are those in which myocardial involvement occurs as one manifestation of a systemic disease. Clinical evidence of myocardial disease may be a significant and dominant finding, but more commonly these features are overlooked or obscured by other non-cardiac manifestations of the disease and not discovered until post mortem examination. The recognized causes of secondary myocardial disease in domestic animals are outlined in Table 1. Although this list seems overwhelming, it should be realized that the majority of these conditions are uncommon and/or infrequently diagnosed. New items are added to the list all the time.
The myocardial effects of this diverse group of diseases is quite varied. Myocardial dysfunction may be the result of multifocal or diffuse areas of myocytolysis or myofiber necrosis, or due to an alteration in myocardial performance without the existence of recognizable microscopic changes. In other secondary myocardial diseases peripheral vascular effects (e.g. systemic hypertension, peripheral vasodilatation, thromboembolism, and shock) may act in concert with alterations in cardiac performance to produce myocardial dysfunction.
Clinical manifestations of the secondary myocardial disease, when present, do not differ significantly from those found in other forms of myocardial disease. Cardiac arrhythmias, congestive heart failure and sudden death are possible events. Generally speaking, the prognosis is poor, unless the responsible underlying multisystem disease can be identified early in its course and is readily controlled by therapeutic measures.
1. Myocardial disease of known or suspected heritability
a. Hereditary cardiomyopathy in Syrian hamsters
b. Various strains of mice with hereditary muscular dystrophy
c. Hypertensive laboratory rats
d. Hereditary cardiomyopathy of turkeys (“Round Heart Disease”)
e. Glycogenesis (glycogen storage diseases) or other inherited myopathies
f. Many types have been documented in animals
-Type I (Von Gienke’s disease) – glucose-6-phosphatase deficiency.
-Type II (Pompe’s disease) – acid maltase or alpha-1,4 glucosidase deficiency, Shorthorn and Brahman cattle, Corriedale sheep, Lapland dogs, Japanese quail
-Type III (Cori’s disease) – amylo-1, 6-glucosidase deficiency). German Shepherd and Akita dogs
-Type VIII – phosphorylase kinase deficiency. Rats and mice.
-Duchene’s muscular dystrophy – Golden Retrievers
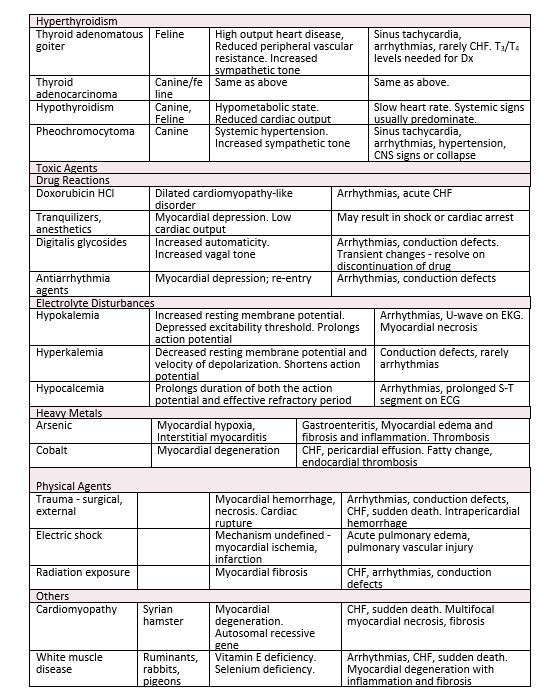
2. Myocardial diseases secondary to nutritional deficiencies
a. Selenium-Vitamin E deficiency
a. Many species affected, including man
b. Myocardial and skeletal muscle necrosis is c consistent finding
d. Etiologic factors include:
-Low dietary levels of selenium, vitamin E and sulfur-containing amino acids
-High dietary concentration of polyunsaturated fats
-Exposure to prooxidant compounds
-Intake of selenium antagonists such as silver salts and other metals
-Frequently called “White Muscle Disease”
b. Copper deficiency – adult cattle
a. Seen in cattle maintained in copper deficient pastures
b. Microscopic findings – extensive myocardial fibrosis
c. Thiamine (Vitamin B1) deficiency – Beriberi Heart Disease
a. Common cause of heart disease in people in undernourished nations
b. Reported in many species of animals – most experimentally produced
c. Hemodynamic changes:
-Increased cardiac output, stroke volume
– Peripheral vasodilatation – reduction in peripheral vascular resistance
d. Taurine deficiency in cats (see Dilated Cardiomyopathy)
3. Myocardial diseases of toxic etiology
a. Cobalt cardiotoxicity
a. Biochemical lesion – blocking of the oxidation of alpha-ketoglutarate and pyruvate by complexes formed between cobalt and sulfhydryl groups of a-lipoic acid.
b. Myocardial energy metabolism is compromised as in thiamine deficiency.
b. Catecholamine cardiotoxicity
a. May occur by increased circulating levels of endogenous catecholamines (as in pheochromocytoma), or by the administration of exogenous catecholamines.
b. Myocardial lesions – multifocal myocardial necrosis, most severe in the left ventricular subendocardium and papillary muscles.
c. Minoxidil (Loniten) cardiotoxicity
a. Vasodilator agent used in humans with refractory hypertension, balding
b. In dogs doses as low as 1 mg/kg result in:
-Severe right atrial hemorrhage with inflammation and fibrosis
-Left ventricular papillary muscle necrosis
d. Doxorubicin (Adriamycin) and Daunorubicin (Cerubidine) cardiotoxicity
a. Antineoplastic drug used primarily in chemotherapy of solid tumors
b. Cumulative toxicity – a dilatative cardiomyopathy-like syndrome with severe CHF. Develops when maximum recommended total cumulative dose exceeds 240 mg/m2; sometimes occurs in breeds predisposed to cardiomyopathy at lower doses than 240 mg/m2.
c. Microscopic myocardial lesions include: sarcoplasmic vacuolization, myocytolysis and hyaline necrosis.
e. Furazolidone cardiotoxicity in poultry
a. Antibiotic used as food additive in poultry feeds
b. Cardiotoxicity reported in turkeys, ducklings and chickens
c. Accidental exposure to excessive amounts of this antibiotic is the usual cause and results in congestive heart failure.
f. Renal failure
a. Associated myocardial necrosis is felt to be a sequel to systemic hypertension
b. Lesions (focal) most severe in left ventricular subendocardium, uremic vasculitis may contribute.
c. Target end organ damage includes LV hypertrophy, glomerulosclerosis and progressive renal failure, retinal hemorrhage or detachment leading to blindness, and hypertensive encephalopathy or CNS stroke or hemorrhage leading to neurologic deficits.
4. Myocardial disease associated with physical injuries
a. Central nervous system lesions
a. Myocardial necrosis and/or hemorrhage
b. Lesions are similar to those resulting from excessive administration of catecholamines
b. Electric shock; defibrillation
a. Focal myocardial necrosis – occurs in areas of high current density
b. Factors increasing severity of necrosis:
-Shocks of high strength (milliamps); Use of small electrodes
-Delivery of multiple shocks
-Frequent shocks with short intervals between
c. Hemorrhagic shock
a. Myocardial lesions are consistently found in experimental hemorrhagic shock and probably are of comparable frequency in naturally occurring shock of various causes.
b. Lesions most pronounced in left ventricular subendocardium and papillary muscles. Subendocardial hemorrhage and microscopic regions of focal necrosis.
c. May be seen in dogs with trauma, GDV, splenic mass, pancreatitis, etc. Is associated with development of ventricular arrhythmias and high cardiac troponin I.
5. Myocardial disease associated with endocrine disorders
a. Diabetes mellitus
a. Myocardial lesions reported only in man and genetically diabetic mice.
b. In man pathologic studies have defined the presence of a microangiopathy involving the intramural coronary arteries. At the present time the mechanism of heart failure in patients with so called diabetic cardiomyopathy must be considered undefined.
b. Hyperthyroidism
a. Cardiac alterations secondary to the hyperthyroid state have been reported both in the clinical setting in man and the domestic cat.
b. Cardiovascular effects are the result of a direct action of elevated circulating levels of thyroid hormones on the heart and peripheral vascular beds, and increased sensitivity of the heart to endogenous catecholamines.
c. Increased cardiac output, heart rate, left ventricular ejection fraction.
d. Decreased peripheral vascular resistance, circulation time
e. Widened pulse pressure
f. Thyroid hormone also stimulates protein synthesis leading to myocardial hypertrophy. This has been recognized microscopically along with increased numbers of mitochondria.
g. Although non-cardiac manifestations of hyperthyroidism usually predominate the clinical picture (i.e., weight loss despite a normal or ravenous appetite, intermittent vomiting, diarrhea, nervousness, open mouth breathing), the cardiac changes frequently are prominent on clinical exam and potentially most life-threatening (tachycardia, arrhythmias, congestive heart failure).
c. Amyloidosis
a. A multisystem disease of undefined pathogenesis characterized by the deposition of amyloid, a fibrillar glycoprotein in various body tissues.
b. Amyloid deposition in the kidneys, liver and other non-cardiac tissues occurs in association with certain chronic infectious, inflammatory and neoplastic diseases; and the resulting dysfunction of these organs is the usual cause of clinical signs in domestic animals.
c. Cardiac involvement is rare, but deposition in the endocardium, myocardium, pericardium, valve leaflets, conduction system and intramural coronary arteries may develop, resulting in a variety of cardiac manifestations and severe cardiac dysfunction.
REFERENCES:
1. Current Veterinary Therapy XIV, Bonagura JD Twedt DC (eds), Saunders Elsevier 2009
Schober KE. Myocarditis Panciera DL. Cardiovascular Complications of Thyroid Disease. CVT XIII on Evolve
2. Canine and Feline Cardiology. Fox PR, Sisson DD, Moise NS.
Cardiac involvement in systemic disease.
