Faculty Syllabus: Pathophysiology & Treatment of Shock
Faculty Syllabus Download
Pathophysiology and Treatment of Shock
John E. Rush, DVM, MS, DACVIM (Cardiology), DACVECC
Cummings School of Veterinary Medicine At Tufts University
Last Updated 04/13/2020
OBJECTIVES
1. Understand the definition of shock, in the context of oxygen delivery (DO2) and tissue oxygen demand (VO2).
2. Differentiate cardiogenic shock, hypovolemic shock, obstructive shock and distributive shock.
3. Be familiar enough with the compensatory mechanisms used to restore blood pressure and cardiac output that you could write an essay on the topic.
4. Be able to describe the classic clinical signs of the different stages of shock, in addition to clinical tests or findings used to diagnose shock.
5. For each of the categories shock in objective 2, discuss treatment in the context of fluids, inotropes, oxygen administration, antibiotics, and steroids.
6. Understand the definitions and causes of the systemic inflammatory response syndrome (SIRS), MODS, bacteremia, sepsis, and septic shock. Be able to explain how treatment of septic shock differs from the aforementioned categories of shock.
SHOCK – WHY DO WE CARE?
Shock is an important and common occurrence in veterinary medicine. While we often don’t talk about it, almost every patient that dies of acute illness, dies with or of shock. Ultimately shock plays a role in fatal illnesses since circulatory failure is part of the final common pathway of cardiopulmonary arrest (CPA).
An understanding of shock is also important since early recognition and appropriate treatment of shock can reverse the sequelae of shock and improve outcome. Unfortunately, we don’t often recognize shock until the late stages, when therapy is frequently ineffective (i.e. its consequences are irreversible).
DEFINING THE SHOCK SYNDROME
Historic definitions
– Gross (1872) – “Manifestations of the rude unhinging of the machinery of life.”
– Blalock (1940) – “Shock is the peripheral circulatory failure, resulting from a discrepancy in the size of the vascular bed and the volume of the intravascular fluid.”
– Simeone (1964) – “Clinical condition characterized by signs and symptoms which arise when the cardiac output is insufficient to fill the arterial tree with blood under sufficient pressure to provide organs and tissues with adequate blood flow.”
Definition at the cellular level
– Shock refers to an imbalance between oxygen delivery to the tissues (DO2) and tissue oxygen consumption/demand (VO2) by the tissues. Specifically, Oxygen delivery (DO2) is less than oxygen consumption/demand (VO2)
– This imbalance occurs due to either or both, decreased oxygen delivery and/or increased oxygen consumption/demand.
– Decreased oxygen delivery may be associated with abnormalities in any of the components that contribute to tissue oxygen delivery.
– The DO2 components are shown below.
Faculty Syllabus Pathophysiology of Shock 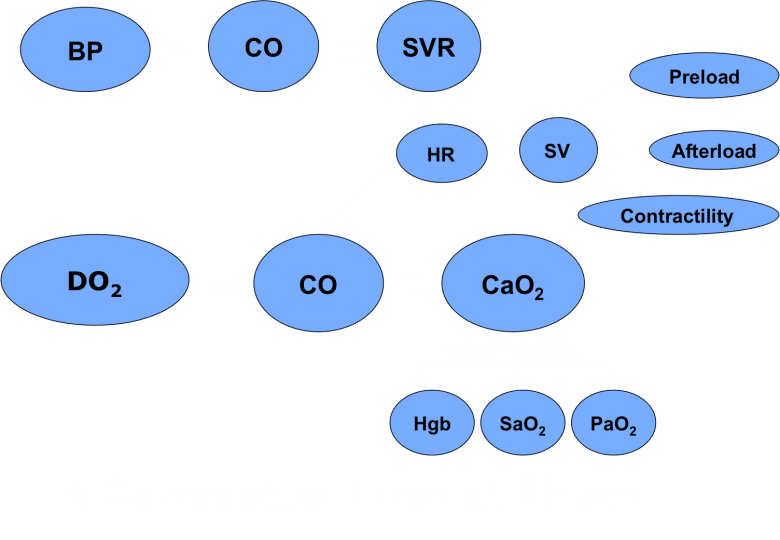
– Components of this equation are explained below.
– DO2 = the volume of oxygen that reaches the systemic capillaries per minute. The two main variables of DO2 are cardiac output (CO) and oxygen content of the arterial blood (CaO2).
– CO = cardiac output; determined by HR (heart rate) and SV (stroke volume).
– SV = stroke volume; determined by preload, afterload and cardiac contractility.
– CaO2 = oxygen content of the arterial blood. Most oxygen is bound to hemoglobin (1.34 x Hgb x SaO2); while a much smaller amount is dissolved in plasma (0.003 x PaO2).
Adequate oxygen delivery requires that each of these components is adequate; or that there is adequate compensation by other components to make up for a deficiency in one. Shock occurs when the circulatory system is unable to compensate for the impairment of any one of these components.
The other variable to consider is oxygen demand by the cells in the body (VO2). Normally, cells receive far more oxygen than they need. But, disease can alter the amount of oxygen required for homeostasis at both the organ level and the most basic cellular level. VO2 can be elevated in a variety of diseases, especially situations associated with a hypermetabolic state and increased oxygen demand, including sepsis, seizures/generalized tremors (e.g. status epilepticus, eclampsia, tremorgenic toxins), heat stroke, and malignant hyperthermia. Shock can result from any of these conditions even though blood flow and oxygen delivery are normal.
Classification Schemes: There are a great variety of classification schemes for shock, and depending upon which text/resource you select, shock might be described or categorized in a different fashion. More than one type of shock may be present in any given patient.
Potential classification schemes in veterinary medicine:
1. Classification of shock based on clinical disease: Cardiogenic shock, Hemorrhagic shock, Septic shock, Endotoxic shock, Anaphylactic shock, Neurogenic shock, etc.
Cardiogenic Shock. Shock due to cardiovascular cause – poor myocardial function, cardiac tamponade, cardiac arrhythmia, etc. See more below.
Hemorrhagic Shock. Essentially the same as hypovolemic shock, as described below, but due specifically to blood loss.
Septic Shock (in large animal medicine people talk about endotoxic shock). Shock caused by bacterial or their components. See more below.
Anaphylactic shock. Anaphylaxis is a systemic manifestation of an acute type 1 hypersensitivity reaction. In the classic case, an antigenic substance causes a response mediated by IgE, resulting in the release of vasoactive mediators by mast cells and eosinophils. Histamine is the prototypic vasoactive mediator that, along with other mediators, results in vasodilation, edema, laryngeal swelling and upper airway obstruction, bronchoconstriction, reduced pulmonary gas exchange, and activation of the complement and coagulation cascades. Venous dilatation, increase in vascular capacity, arteriolar dilatation and hypotension with reduced venous return are contributing factors in the development of hypovolemia and hypotension. Hypotension, vomiting, diarrhea, ataxia, prostration, dyspnea, coma and death can occur in hours (sometimes within minutes) if not treated. Treatment involves administration of 1) Epinephrine (first and foremost – membrane stabilizer as well as other actions), 2) oxygen, 3) removal of the underlying cause, and maybe anti-histamines, anti-inflammatories, fluids, or other vasopressor agents.
Neurogenic shock has been associated with deep anesthesia, depression of the vasomotor center, spinal anesthesia, spinal cord or CNS trauma, and prolonged ischemia of the vasomotor centers. It occurs due to abrupt loss of vasomotor tone, a marked increase in vascular capacity and “pooling” of blood volume in the periphery. Bradycardia may be due to increased CNS pressure (i.e. Cushing’s reflex).
2. Classification of shock based on volume status: Normovolemic vs. Hypovolemic vs Hypervolemic
a. Is blood volume normal?
b. Or is blood volume low and the animal needs fluids, colloids or blood products
c. Or is there too much blood volume (e.g., CHF) and diuretics are indicated
3. Classification of shock based on the major circulatory disorder.
Cardiogenic shock is caused by cardiac dysfunction (i.e. failure of the pump).
Hypovolemic shock is caused by loss (often rapid, but sometimes just GI fluid losses from diarrhea) of intravascular fluid. Included in this group is hemorrhagic shock.
Obstructive shock is caused by physical obstruction of blood flow. An example might be GDV, where the dilated stomach compresses the vena cava and prevents return of venous blood flow to the heart.
Distributive shock (also called vasogenic shock) is caused by a loss of normal distribution (i.e. maldistribution) of blood flow through the vascular system. Examples: Lack of systemic arteriolar tone means low blood pressure and lack of venous tone means fluid pools in the veins and does not return to the heart, and this happens in advanced septic shock.
CLINICAL SIGNS OF SHOCK
The clinical signs of shock can include tachycardia, tachypnea, weak arterial pulses, altered MM color or capillary refill time, reduced urine output, cool limbs/peripheral tissues, and a number of other clinical findings, which can vary from animal to animal. There are also species differences, as cats can have a slow to normal heart rate, especially those that are hypothermic, and the “hyperdynamic” findings in shock (injected membranes and rapid CRT) are much less common in the cat. A brief list of possible physical exam findings associated with shock includes:
1) Tachycardia
2) Tachypnea
3) Pale mucus membranes (MM) with delayed capillary refill time (CRT)
4) Brick red MM with brisk CRT (e.g. < 1 second) in early septic shock
5) Cool limbs
6) Oliguria
7) Weak arterial pulses (sometimes bounding in early septic shock)
8) Reduced level of consciousness
9) Lactic acidosis, altered based excess/base deficit
10) Hypotension
11) Coagulopathy – can lead to disseminated intravascular coagulation (DIC) or organ thrombosis
12) Fever or hypothermia
These clinical signs result from reduced blood flow or tissue perfusion, and the body invokes a number of compensatory mechanisms to counter these effects. The sympathetic nervous system is activated, as is the renin-angiotensin system and ADH and a number of other compensatory mechanisms. The end result of these compensatory mechanisms is to increase cardiac output or tissue perfusion via altered heart rate, vascular tone, inotropic vigor, sodium and water retention, and other mechanisms. These are explored further below:
Decreased blood volume is sensed by both baroreceptors and chemoreceptors. Decreased venous return to the heart results in decreased SV and decreased CO. High pressure baroreceptors sense decreased arterial pressure, while low pressure baroreceptors sense decreased atrial volume. These baroreceptors stimulate the medullary cardiovascular control center, resulting in a sympathetic response. The sympathetic nervous system response includes increased activity and release of circulating catecholamines (notably norepinephrine and epinephrine). This results in an increase in heart rate and an increased inotropic state (i.e. increased contractility), which lead to increased CO. Additionally there is vasoconstriction (both arteriolar and venous) to help maintain / restore BP. Blood pressure is increased at the expense of certain less critical tissues and hence there is reduced perfusion in cutaneous, renal, splanchnic, muscular, portal-hepatic and mesenteric beds (i.e. blood is shunted away from the organs to maintain vital organs such as the brain and heart). The chemoreceptors sense alterations in tissue or blood hypoxemia, hypercapnia and acidosis, which result from decreased tissue perfusion. In addition, central chemoreceptors are stimulated by decreased pH of the ECF. Again chemoreceptor stimulation results in stimulation of the medullary cardiovascular control center and a sympathetic response. The renin-angiotensin-aldosterone (RAAS) system is stimulated, resulting in arteriolar and venous constriction and renal conservation of salt and water in an attempt to maintain blood volume.
Early shock may manifest as mild tachycardia and tachypnea, “brick red” mucus membranes, and a brisk capillary refill time (CRT) e.g. < 1 second – these latter 2 findings are most frequently seen in the early stages of septic shock. This is a hypermetabolic state that cannot be maintained indefinitely, but this stage is more likely to respond favorably to specific therapy (e.g., fluid therapy, antibiotics if appropriate, etc.). As shock progresses there is redistribution of blood flow to the vital organs (heart and brain), and features such as cool limbs, oliguria, membrane pallor or delayed capillary refill time, weak pulses, and reduced level of consciousness may be noted. Oxygen delivery is reduced to the peripheral and splanchnic organs, VO2 becomes dependent on DO2, and anaerobic respiration results in the development of a lactic acidosis and can result in tissue cell death. Tachycardia and tachypnea continue. Tachypnea is at least in part a compensatory response (i.e. hyperventilation and respiratory alkalosis compensate for metabolic acidosis). Blood pressure is usually low. A coagulopathy often develops and can lead to disseminated intravascular coagulation or organ thrombosis.
As shock progresses autoregulation ultimately fails, and there is loss of sympathetic control and the associated chronotropic and inotropic responses. This is the terminal stage of shock and is often refractory even to aggressive therapy. Cardiac arrest is imminent. Clinical signs are associated with complete cardiovascular collapse and include severe tachycardia or bradycardia, decreased heart sounds, weak or absent femoral pulses, grey membranes or absent CRT, severe hypotension, stupor, coma and organ failure.
CLINICAL AND PATHOPHYSIOLOGIC FEATURES OF SHOCK BY CAUSE
CARDIOGENIC SHOCK can theoretically occur secondary to any cardiac disease including valvular disease, myocardial disease, cardiac arrhythmias, pericardial disease, or myocardial infarction. In small animal medicine cardiogenic shock is often seen secondary to DCM with concurrent left-sided congestive heart failure, and in cats with hypertrophic cardiomyopathy. Cardiac tamponade (pericardial effusion), tension pneumothorax, ruptured chordae tendineae, myocardial toxins, myocardial injury from sepsis, and pulmonary thromboembolism are additional causes. These diseases cause shock by causing low cardiac output. In some cases this is associated low HR (e.g. third degree AV block). However in the majority of cases it is associated with low stroke volume, secondary to abnormalities of preload, afterload or contractility.
Clinical signs associated with cardiogenic shock can include cyanosis, respiratory distress, pulmonary crackles, tachycardia, a cardiac murmur or gallop, cardiac arrhythmias, and weakness. One of the main differences in clinical findings from those identified for shock above is the finding of distended jugular veins and elevated CVP and/or pulmonary edema. Many cases of cardiogenic shock are accompanied by congestive heart failure and significant fluid retention, and this is an important feature from a therapeutic perspective as fluid therapy is typically contraindicated in individuals with cardiogenic shock and concurrent heart failure.
HYPOVOLEMIC SHOCK is arguably the most common cause of shock in veterinary medicine, and is treated as outlined below with fluids, colloids or blood products. Hypovolemia associated with blood loss (AKA hemorrhagic shock) is associated with an actual loss of intravascular volume caused by either internal or external blood loss. Examples include hemoabdomen, hemothorax, or blood loss from a traumatic arterial laceration. Hypovolemia not associated with blood loss involves loss of plasma volume from the vascular space or pooling of blood or failure to retain fluid in the body. Examples include severe dehydration caused by vomiting and diarrhea (e.g., viral or bacterial diarrhea), burns, third-spacing of fluid, as which occurs with pleural or peritoneal effusion, diuresis with excessive urinary losses, or Addison’s disease.
Classic clinical findings of shock are present in the setting of hypovolemia. Other clinical signs reflect the underlying cause. Since loss of circulating blood volume is the precipitating cause for hypovolemic shock this is the main deficit that needs to be corrected with treatment (i.e., give fluid volume or blood products).
OBSTRUCTIVE SHOCK is associated with an obstruction within the vascular system that prevents blood flow from reaching the tissues. Obstructive shock is less common than cardiogenic or hypovolemic shock. Examples of obstructive shock include gastric dilatation and volvulus (GDV), heartworm caval syndrome (heartworms blocking flow across the tricuspid valve and in the right heart; also considered a cardiac cause), pericardial effusion (impaired right ventricular filling; also a cardiac cause), and pulmonary thromboembolism.
DISTRIBUTIVE SHOCK is associated with maldistribution of blood flow that some tissues do not receive adequate oxygen delivery. Distributive shock can overlap with other shock classifications. Distributive shock is most commonly caused by severe systemic vasodilation and pooling of blood in peripheral capillary beds, away from vital organs. It is classically seen associated with septic shock, but maldistribution can happen with anaphylaxis and traumatic shock as well. Maldistribution of blood flow is difficult to quantify in a clinical setting, but blood lactate and based excess/base deficit are typically abnormal.
SEPTIC SHOCK results from the host response to bacterial or fungal pathogens and is one of the most difficult forms of shock to treat. Early recognition is a key to successful outcomes, and this a number of terms have been devised to get people thinking about whether septic shock may be present in any individual animal, and get professionals talking with similar terms about various clinical scenarios. Despite significant advances in our understanding of the pathophysiology of sepsis, identifying sepsis early is still a challenge. Current evidence suggests that early detection and evidence-based treatment strategies dramatically reduce morbidity and mortality in sepsis (e.g., fluids, antibiotics, source control, etc.). So, some more definitions!!
SIRS, SEPSIS, AND MODS: Please learn these definitions in BOLD!
SIRS – The systemic inflammatory response syndrome (SIRS) occurs due to an imbalance between pro- and anti-inflammatory mediators in response to an insult. SIRS can be the result of an infectious insult (sepsis) or a non-infectious insult such as trauma, cancer, and various sterile inflammatory diseases such as pancreatitis, heat stroke, or snake envenomation. While inflammation is a normal response to these insults to the body, in SIRS the inflammation is no longer contained locally, and now affects the whole body. When inflammation becomes systemic, then it has the potential to adversely affect the host, and it should trigger your “concern” area that this is an animal with serious illness.Inflammatory mediators include cytokines, chemokines, vasoactive substances and mediators of coagulation, and these mediators are not specific for sepsis. Prototypic cytokines include tumor necrosis factor (TNF), interleukin (IL)-1 and IL-6; these cytokines contribute to the development of fever, cardiovascular instability, increased vascular permeability and acute phase protein synthesis. Chemokines, notably IL-8 (new nomenclature CXCL-8) induce neutrophil chemotaxis to sites of inflammation. Vasoactive substances produced in response to pathogen recognition include nitric oxide, an extremely potent vasodilatory substance. Stimulation of inflammation also stimulates coagulation; one of the earliest steps in the interplay between these two systems is the intravascular expression of tissue factor (TF), which can trigger DIC. While inflammation is a normal response to these insults to the body, in SIRS the inflammation is no longer contained locally, and now affects the whole body. When inflammation becomes systemic, then it has the potential to adversely affect the host, and it should trigger your “concern” area that this is an animal with serious illness.
SIRS is a clinical diagnosis, that you can make on the basis of the TPR (temperature, pulse and respiratory rates), and white blood cell count (from your complete blood count). While specific definitions are published there is no consensus in veterinary medicine, and what denotes SIRS varies between species. Essentially SIRS is considered present if the animal fulfills 2 or 3 out of the 4 following SIRS criteria:
- Abnormal temperature (either fever or hypothermia)
- Abnormal heart rate (either tachycardia or bradycardia)
- Tachypnea
- Leukocytosis, leucopenia or a significant left shift (bands)
Identification of these clinical criteria in a patient should then prompt a search for the cause / underlying disease; either non-infectious or infectious (and aggressive treatment).
Bacteremia: The presence of bacteria in the bloodstream – can be seen in sepsis, but also occurs with vigorous brushing of teeth and many surgical procedures.
Endotoxemia: Presence of endotoxin in the bloodstream – can happen with or without sepsis. Talked about more in horses than other species.
Sepsis: The animals has SIRS (criteria above fulfilled) and the animal has a confirmed (or highly suspected) infection (e.g., from cytology, culture, histopath, PCR, etc). The source of infection may or may not be immediately obvious, and diagnostic testing may include bloodwork, thoracic radiographs, abdominal ultrasound, centesis or aspiration for cytology and culture, urinalysis, etc. Sepsis (the systemic inflammatory response to infection), is associated with high morbidity and mortality. Despite significant advances in our understanding of the pathophysiology of sepsis, identifying sepsis early is still a challenge. Current evidence suggests that early detection and evidence-based treatment strategies dramatically reduce morbidity and mortality in sepsis.
Severe Sepsis: Sepsis with one or more organ dysfunctions. Examples include:
- Renal dysfunction (rise in [Creatinine] > 0.5mg/dL)
- Cardiovascular dysfunction (myocardial dysfunction or requirement for pressors)
- Respiratory dysfunction (requirement of oxygen or mechanical ventilation)
- Hepatic dysfunction (T. bili >0.5mg/dL)
- Coagulation dysfunction, DIC (thrombocytopenia, prolonged PT, PTT, etc.)
- Gastrointestinal dysfunction (vomiting, regurgitation, ileus, constipation, diarrhea)
- Endothelial dysfunction (vascular leak with edema formation and low albumin)
- Laminitis (horses)
Multiple Organ Dysfunction Syndrome: The presence of two or more of these organ dysfunctions is referred to as multiple organ dysfunction syndrome (MODS); this can occur due to either sepsis or non-infectious causes.
Septic shock: Severe sepsis with hypotension that is non-responsive to intravascular volume expansion (i.e. sepsis requires a vasopressor to maintain blood pressure).
Not surprisingly, mortality increases dramatically as the patient progresses from sepsis to severe sepsis to septic shock. Another important part of managing the septic patient is prompt initiation of appropriate, aggressive treatment. IV antibiotic therapy should begin immediately. In general, multiple antibiotics are administered initially while cultures are pending (e.g., an aminoglycoside and ampicillin +/- a third antibiotic)
TREATMENT OF SHOCK
PRINCIPLES
Shock is a dynamic, multisystemic disorder. Animals with shock change rapidly, and thus therapy is not “a set of specific things to do in each case” but rather is dictated by serial monitoring of patient parameters and assessing response to treatment.
TREAT THE UNDERLYING CAUSE
Addressing the underlying disease as promptly as possible is vital for the successful management of shock. Sometimes however this is easier said than done, especially when the etiology in uncertain. For hypovolemic shock, it is vital to stop any further loss of blood volume. In the setting of hemorrhage (either internal or external), this means controlling the hemorrhage and usually requires surgery (e.g. splenectomy for a bleeding splenic mass causing hemoabdomen). In order to treat obstructive shock, the obstruction to blood flow must be removed or resolved as best as possible (e.g., for GDV this means reducing the size of the dilated stomach by trocarization or passage of an orogastric tube). Caval syndrome is treated by removing the adult heartworms from the right heart to restore normal flow of blood into the heart. In cardiac tamponade, emergency pericardiocentesis is indicated. Septic shock requires identification and management of the original source of sepsis (e.g., exploratory celiotomy for septic peritonitis). For anaphylactic shock, removal of the initiating factor (remove bees, stop giving antibiotics, etc.).
STABILIZATION – INITIAL TASKS
1. Secure the airway; supplemental oxygen as needed (nasal lines, O2 cage, etc.); if required intubation and mechanical ventilation.
2. Cardiac and circulatory improvement – Maximize oxygen delivery to tissues (DO2) – see algorithm below
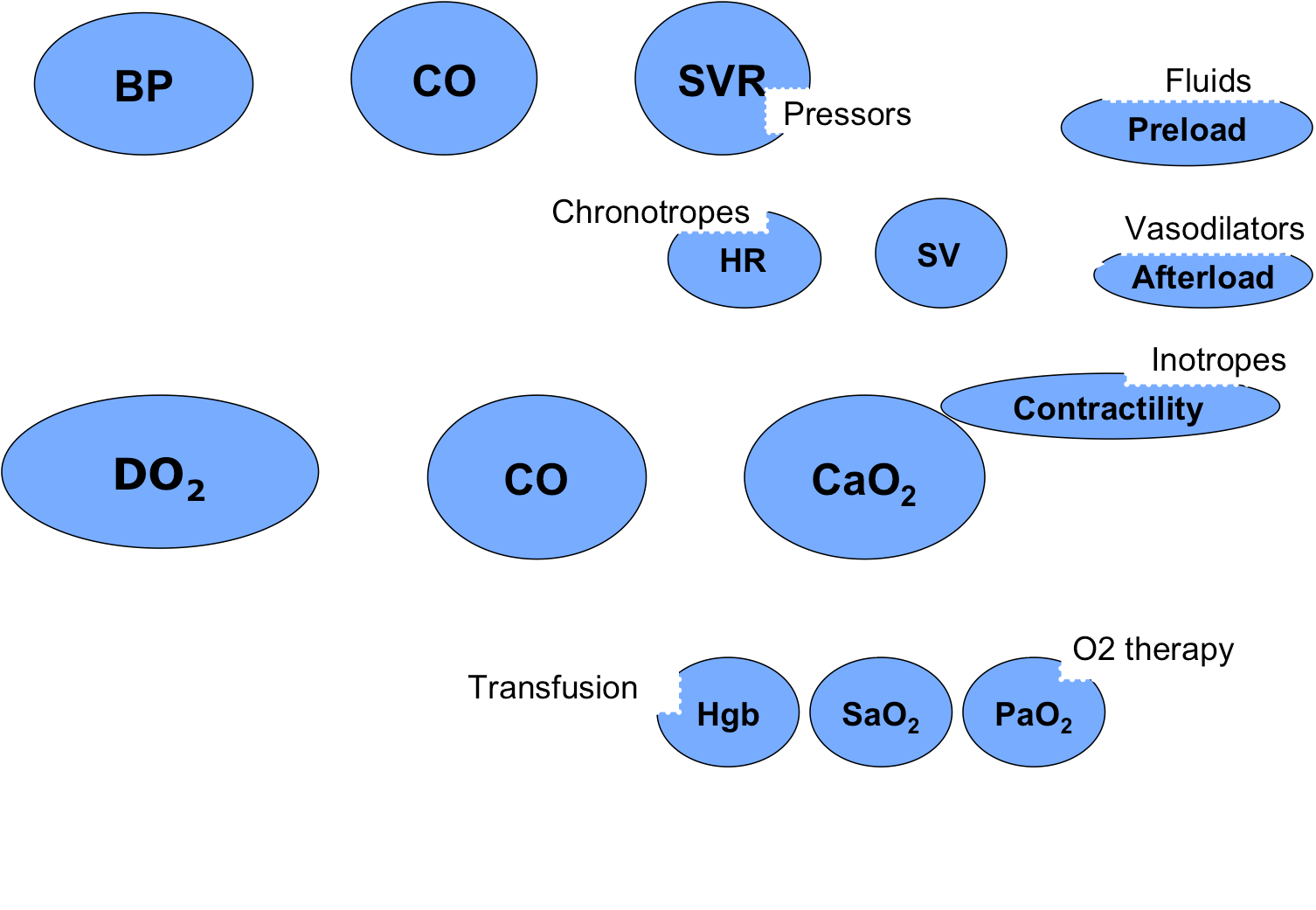
The key of treatment of shock is to resuscitate to end-points. The parameters that we use to guide resuscitation are the same as those used to identify shock in the first place. These include level of consciousness, mm color, CRT, HR and pulse quality, blood pressure, blood lactate, and base deficit. Resuscitation to end-points means that we give the minimum amount of fluid needed to restore parameters to an acceptable level.
END-POINTS OF RESUSCITATION – A GUIDE:
– Improved level of consciousness (responsive, alert is ideal)
– Improved heart rate (ideally to normal range)
– Improved arterial pulse quality
– Improved mm color (at least light pink), and normalized CRT
– Normothermia
– Blood Lactate < 1.5 to 2.0 mmol/L
– Normal blood pressure (SBP > 90 mmHg, MAP >60mmHg, ideally SAP > 110 mmHg)
– CVP ≥ 5 cmH2O (especially with distributive shock; before reaching for vasopressors)
– PCV 20-25%
– SpO2 > 90% (bare minimum or mechanical ventilation is needed)
– pH > 7.25, Base excess –4 to +4 mE/L
– Urine output at least 1.5 to 2 ml/kg/hour
TREATMENT OF SHOCK – FLUID THERAPY is usually step 1
Intravenous fluids (IVF) to increase preload and hence SV and CO, are the mainstay of therapy of shock, with the exception of cardiogenic shock in which IVF are usually contraindicated.
We often refer to a “shock dose” of fluids; however, fluids are not dosed like other drugs, and there is no standard dose for shock. A “shock dose” represents a blood volume (about 8% of body weight), and it is suggested merely as a guide (generally of the upper limit of IVF volume to be administered in an hour).
In general fluid resuscitation from shock is achieved with isotonic crystalloids, but on rare occasion hypertonic crystalloids and/or synthetic colloids can be used. In hemorrhagic shock, administration of packed red blood cells and fresh frozen plasma can be mainstays of therapy, but crystalloids are still usually the first step in veterinary species. Fluids administered for shock are typically given as a bolus (often about 20 ml/kg), over 5-15 minutes, generally through a short, large bore catheter. Depending upon animal size and fluid needs, the bolus can be administered by gravity flow, with a pressure bag, using a fluid pump, or a syringe pump.
Isotonic crystalloids (e.g. LRS, Normosol-R, Plasmalyte-148 and 0.9%NaCl) are generally the first line fluid for the treatment of shock. The “shock dose” of isotonic crystalloid is 80-90mL/kg/hr for dogs and most mammalian species, and 60mL/kg/hr is usually recommended for cats. This shock dose can be divided into “aliquots” and given serially while monitoring response to treatment. For example, some clinicians choose to administer 20mL/kg given 5-15 minutes and then reassess heart rate, membrane color and CRT, +/- blood pressure or lactate etc. If the animal still has clinical signs of shock, this dose can be repeated, and so on, until end-points of adequate resuscitation are reached, or until a shock dose of fluids has been administered. Cats also seem to be more prone to fluid overload (pulmonary edema or pleural effusion) when given large volumes of crystalloid fluids.
Hypertonic saline (7.5% NaCl) is sometiems used, especially for very large animals (e.g., horses, Great Dane), as part of the initial fluid resuscitation from shock. It may also be used in some cases of traumatic brain injury. It has a very potent (albeit short-lived) ability to expand the intravascular space (by ~3x its own volume within 30 minutes). It is given as a single dose of 3-5mL/kg slow IV over ~ 10 minutes. Side effects can include arrhythmias, hypernatremia and hyperosmolality. Hypertonic saline is essentially ALWAYS followed by aggressive crystalloid resuscitation or shock will return.
Albumin or Synthetic colloids (e.g. Hetastarch, Dextrans 70 and Voluven) can be used as an adjunct to isotonic crystalloid resuscitation. However, their use is not recommended alone, and they have largely been abandoned in the human medical field due to concerns about renal injury and worse clinical outcomes (compared to crystalloids). The “dose” of synthetic colloids is 20mL/kg in dogs and 10mL/kg in cats. Like crystalloids, this can be divided and given as 2- 5mL/kg aliquots at a time. By increasing the colloid oncotic pressure, they draw fluid into the intravascular space, increasing the intravascular space by about twice the volume infused. In contrast to hypertonic saline, its effect lasts a significant period of time (i.e. >4-6 hours). Care should be taken with colloid use in animals prone to fluid overload (e.g. cats, animals with underlying cardiac or renal disease). Synthetic colloids are perhaps most indicated in animals with low colloid oncotic pressure (e.g., low albumin).
Blood products such as packed red blood cells or fresh frozen plasma is indicated in the setting of severe anemia, active hemorrhage and/or coagulopathy. Administration of albumin (either human serum albumin or canine serum albumin) may be considered in animals with low albumin (low COP), however especially with human products there is significant risk of anaphylaxis and/or delayed type hypersensitivity reactions.
Continuous re-evaluation of the patient is vital as fluid needs can change quickly as the disease changes minute-to-minute in some of these cases. Excessive fluids lead to cellular edema (and hinder oxygen transport into cells) and can lead to fluid overload causing CHF with pulmonary failure. Inadequate IV fluid therapy leads to persistence of the hypovolemic status, decreased tissue perfusion, and encourages the continued downward spiral toward multiorgan failure and death.
TREATMENT OF FLUID UNRESPONSIVE SHOCK – VASOPRESSORS
In some shock states, increasing preload alone is inadequate to resolve shock. If shock persists despite a “shock dose” of fluids, then administration of a vasopressor agent to increase systemic vascular resistance is often indicated. Vasopressors are given IV as continuous rate infusions (CRIs) with very close monitoring of the patient and blood pressure (ideally with direct / invasive blood pressure monitoring). Norepinephrine is often used as the first line vasopressor to restore blood pressure (and tissue perfusion). Vasopressin and epinephrine are much more potent vasopressors, but have the potential to increase morbidity associated with reduced splanchnic perfusion and deleterious beta effects of epinephrine (i.e. increased cardiac work). Additionally, vasopressors can cause severe cardiac arrhythmias so ECG monitoring is recommended.
TREATMENT OF CARDIOGENIC SHOCK
Cardiogenic shock is somewhat of a unique situation. These patients typically do not have hypovolemia/decreased preload, and thus do not get fluids. Instead, when CHF is present, this is the one indication for diuretics in animals with shock. The underlying cardiac cause should be addressed with examples being:
– CHF (e.g., pulmonary edema – give oxygen and furosemide)
– Bradycardia (e.g. third-degree AV block – pacemaker required)
– Tachycardia (e.g., ventricular tachycardia – give lidocaine)
– Impaired contractility (e.g. DCM – give pimobendan or dobutamine)
– Cardiovascular obstruction (e.g., cardiac tamponade – pericardiocentesis)
TREATMENT OF SEPTIC SHOCK
Prompt initiation of treatment is a key to management of septic shock. Fluids and usually multiple antibiotics should be administered within the first hour of recognition of septic shock. In people with septic shock, delays in the administration of appropriate IV antibiotics have been shown to dramatically increase mortality. IV antibiotic therapy should ideally begin immediately. In our hospital we have “sepsis kits” containing doses of IV antibiotics that are readily available to the doctors and nurses in our ER, such that there is minimal delay from ordering to administering antibiotics.
ADJUNCTIVE TREATMENTS FOR SHOCK
Depending upon the cause of shock, other therapies may be possibly indicated. Metabolic acidosis, usually a high anion gap / lactic acidosis, is common in shock, but in most cases treatment of shock will improve the situation and sodium bicarbonate is not needed, and can have adverse effects. Heparin or other anticoagulants may be indicated in situations where thrombosis or DIC are suspected. In certain specific situations, steroids may be indicated (Addison’s disease; MAYBE vasopressor-dependent septic shock).
MONITORING THE PATIENT WITH SHOCK
See “END-POINTS OF RESUSCITATION” above. Close monitoring is vital to pick up any changes in the patient so as to intervene as soon as possible and hopefully prevent any further deterioration.
Monitoring may include:
– Temperature (since a fever may indicate development or recurrence of sepsis, and hypothermia can be a poor prognostic sign if it persists despite treatment)
– Heart rate (sinus tachycardia may indicate ongoing shock; bradycardia concerning for impending cardiopulmonary arrest)
– Respiratory rate and effort, SpO2 (tachypnea, increased effort or decreasing SpO2 may indicate respiratory dysfunction)
– Blood glucose (since hypoglycemia may indicate bacterial consumption, sepsis and hyperglycemia can be concerning in neurogenic injury)
– Blood lactate (since hyperlactatemia may indicate hypoperfusion / shock)
– Markers of kidney function (Creatinine, urine output, urine specific gravity)
– Markers of hepatobiliary function (evidence of icterus – skin, urine, color of serum when running PCV/TS)
– Other markers of cardiovascular function (pulse quality, blood pressure, echo parameters, cardiac arrhythmias)
