Student Syllabus Download
CARDIOMYOPATHIES
Created by V’22 Cardio Group modified from Dr. John Rush
Last Updated: 05/17/2020
Primary Myocardial Disease – Cardiomyopathy
Cardiomyopathy: disease of myocardial structure or function that are independent of valvular disease, congenital heart disease, pericardial disease, and pulmonary or systemic hypertensive disorders
- MOST important cardiac disease in cats
- Second most common cause of congestive heart failure (CHF) in dog
- Reported in many species (horses, ferrets, cattle, etc.)
- Can be classified based upon:
- Anatomic or functional abnormalities
- Dilated cardiomyopathy
- Hypertrophic cardiomyopathy
- Arrhythmogenic right ventricular cardiomyopathy
- Restrictive cardiomyopathy
- Pathogenesis or etiology
- If etiology is unknown → primary cardiomyopathy (i.e. idiopathic dilated cardiomyopathy)
- If etiology is identified → secondary cardiomyopathy (i.e. doxorubicin-induced cardiotoxicity = doxorubicin cardiomyopathy)
- Common clinical manifestations of cardiomyopathy
- CHF
- Cardiac arrhythmias
- Episodic weakness
- Syncopal episodes
- Thromboembolism
- Sudden death
- Anatomic or functional abnormalities
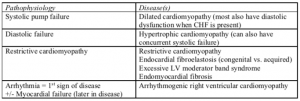
Pathophysiology of cardiomyopathies
Dilated cardiomyopathy (DCM)
- General disease characteristics
- Gross findings:
- Dilation of all four chambers (biventricular enlargement with biatrial enlargement)
- Thinning of ventricular free walls & septum
- Flattening of papillary muscles
- Increased circumference of AV valves due to dilation of chambers
- Microscopic findings:
- Mild endocardial fibrosis
- Mild interstitial fibrosis and edema
- Focal areas of myocytolysis +/- mild mononuclear infiltrate
- Result = Myocardial systolic dysfunction (also the end result of many myocardial pathologies – i.e. taurine deficiency in the cat)
- Gross findings:
- Canine DCM
- Common Signalment
- Giant breeds predisposed, especially Irish Wolfhound, Great Dane, St. Bernard, German Shepherd, Doberman, Boxer
- Males predominate, avg. 4-6 years
- Historical complaints
- Dyspnea
- Cough
- Syncope
- Lethargy
- Weight loss
- Anorexia
- Abdominal distension
- Physical exam (PE) findings
- Pulmonary crackles
- Jugular vein distension
- Hepatosplenomegaly
- Ascites
- Diminished heart and/or lung sounds (if pericardial and/or pleural effusion from CHF)
- Murmur corresponding to mitral or tricuspid valve regurgitation due to chamber dilation
- Gallop (usually S3)
- Cardiac arrhythmias with pulse deficit
- Mucous membrane pallor
- Weak pulses
- ECG
- Left ventricular enlargement (LVE) pattern
- Left atrial enlargement pattern (P-mitrale)
- Arrhythmias common, especially of ventricular origin and atrial fibrillation
- Thoracic radiographs
- Generalized cardiomegaly = MOST common finding
- Pulmonary venous distension
- Interstitial – alveolar pattern, corresponding to pulmonary edema with Left heart failure
- Pleural effusion or ascites with Right or Biventricular failure
- Clinical pathology
- Elevated BUN/creatinine – prerenal azotemia due to inadequate cardiac output
- Can also see this with initiation of diuretic therapy, along with mild hypoproteinemia, hypokalemia
- Elevated liver enzymes – chronic passive hepatic congestion
- Increased NT-proBNP
- +/- Increased cardiac troponin I
- Elevated BUN/creatinine – prerenal azotemia due to inadequate cardiac output
- Echocardiography
- Left ventricular dilation with markedly decreased contractility
- Reduced thickness of the septum and LV free wall
- Left atrial enlargement
- RV and RA dilation
- Increased E point septal separation on M-mode (distance between the anterior mitral valve leaflet and the interventricular septum when mitral valve is open during diastolic filling)
- Treatment
- Pimobendan
- Diuretics (furosemide)
- ACE inhibitors (lisinopril, benazepril, enalapril)
- Low salt diet
- Exercise restriction
- Anti-arrhythmic medications (as needed)
- Dobermans & other dogs in cardiogenic shock: may benefit from dobutamine +/- combination with sodium nitroprusside by CRI for 2-3 d.
- Common Signalment
- Feline DCM
- Used to account for ~ 40% of feline myocardial disease. Now, significantly lower since the discovery of dietary taurine deficiency in cats, leading to DCM
- Common signalment
- 7-8 years
- Siamese, Burmese, Abyssinian may be predisposed
- Historical complaints: often acute (1-3 d.) development of vague clinical disease
- Anorexia
- Vomiting
- Lethargy
- Dyspnea
- Lameness from arterial embolization
- PE findings
- Hypothermia = common
- Very depressed
- Soft murmur or cardiac gallop (S3 usually)
- Pulmonary crackles
- Muffled heart and/or lung sounds (due to pericardial and/or pleural effusion) – common
- Weak apex beat and femoral pulses
- Evidence of thromboembolism
- May present in cardiogenic shock
- ECG: may have normal sinus rhythm
- Bradycardia (esp. if hypothermic)
- LVE pattern
- Various arrhythmias, esp. VPCs
- Thoracic radiographs
- Generalized cardiomegaly & rounding of cardiac apex
- Difficult to distinguish cardiac silhouette due to pleural effusion
- Pulmonary venous distention or pulmonary edema
- +/- Hepatomegaly
- Clinical pathology
- Pre-renal azotemia
- Elevated liver enzymes (ALT, AST – hepatic congestion_
- IF thromboembolic disease is present – coagulation abnormalities, may be compatible with DIC
- IF taurine deficient – low plasma taurine levels (<20 nM/mL)
- Echo findings: similar to dog
- Generalized cardiomegaly with markedly increased end-diastolic & end-systolic LV dimensions
- Decreased fractional shortening
- Chamber wall thinning with LA and RV dilation
- Treatment
- Pimobendan
- Furosemide
- ACE inhibitor (enalapril)
- +/- Nitroglycerin (useful for acute pulmonary edema)
- Supplemental taurine (250 – 500 mg/day)
- Equine DCM
- Atrial fibrillation in some
- May see peripheral edema if CHF is present
- May result from monensin toxicity
- Treatment is limited to furosemide and digoxin, due to the high expense of other treating large animals with other cardiac medications
- Mixed information regarding the effectiveness of ACE inhibitors
- Generally, rarely do well with therapy
Canine Cardiomyopathy Syndromes
Diseases in some breeds of dogs have specific clinical syndromes. This is likely due to different gene mutations accounting for the cardiomyopathy in each breed.
- Giant Breed Cardiomyopathy: Irish Wolfhound, Great Dane, St. Bernard, German Shepherd)
- Left or biventricular heart failure
- Common rhythm diagnosis = atrial fibrillation
- Sudden death possible
- Prognosis
- If CHF is present, usually < 6 mo.
- If discovered and treated early: possibly 1-2 yr.
- If arrhythmia is primary presenting problem: possibly 1-2 yr.
- Doberman Cardiomyopathy: 2.5 – 15 yr. (avg. = 6.5 yr.). 50 – 65% of Dobermans > 4 yr.
- Radiographs: LA & LV enlargement
- Severe (+/- acute) pulmonary edema
- ECG
- LV or biventricular enlargement pattern
- Ventricular arrhythmias
- RBBB pattern common (negative QRS in lead II)
- Sudden death is common (probably associated w/ ventricular arrhythmias)
- Cardiogenic shock may occur if pulmonary edema
- Poor prognosis (most < 8-10 wk.) after onset of clinical signs
- Occult cardiomyopathy markers
- Ventricular arrhythmia
- Dilation of LV cavity
- Genetic mutation identified, allows screening of healthy dogs to identify who might get this disease
- Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) = Boxer Cardiomyopathy, 0.5 – 15 yr. (avg. = 8 yr.)
- Also seen in English Bulldogs
- Arrhythmias common, may not result with antiarrhythmic therapy
- VPCs, frequently with LBBB pattern (positive QRS in lead II)
- Radiographs and echo may be normal in early stages
- Boxer breed = more likely to have ventricular arrhythmias → syncope
- Clinical categories associated with prognosis:
- Category I: asymptomatic w/ arrhythmias ~ 2+ yr. survival
- Category II: arrhythmias + syncope ~ 1 – 2 yr. survival
- Category III: CHF w/ arrhythmias ~ 2 – 10 mo. Survival
- Sudden death possible
- Histology
- Loss of myocytes in the RV, replaced with fat and fibrous tissue
- +/- Myocarditis
- Holter monitoring for 24 hr. assists with screening of asymptomatic dogs
- Genetic mutation identified to screen healthy Boxers who might get this disease
- English and American Cocker Spaniel Cardiomyopathy: 10 mo. – 12 yr. (Avg. = 5 – 9 yr.)
-
- ECG
- R wave > 3.0 mV
- APCs common
- Generalized cardiomegaly w/ pulmonary edema
- Myocardial disease +/- endocardiosis
- Some of these dogs live asymptomatic for a long time
- Some of these dogs have low taurine levels → supplementation with taurine and carnitine may help them liver longer (6+ mo.) and have improvements in cardiac size and function
- ECG
- Myocardial carnitine deficiency: described in some dogs with DCM
- Can increase levels with oral supplementation
- In general, dogs with myocardial carnitine deficiency and DCM do not revert to normal after supplementation – although some may exhibit clinical improvement
- Some dogs with DCM (cocker spaniel, golden retriever, Newfoundland) have low plasma taurine concentrations → partial clinical response with taurine supplementation +/- reduced need for cardiac medication
Hypertrophic Cardiomyopathy
- Canine: very rare, most recognized at necropsy
- Predominant in large breeds (German shepherd, inherited in German shorthaired pointer)
- Conduction abnormalities possible – third degree AV block
- CHF, sudden death
- Feline: most common myocardial disease in cats! Esp. young – middle aged (Avg. = 6 – 9 yr.)
- Genetic mutation in Maine Coon cats – myosin binding protein C
- Sarcomeric mutation likely in other breeds (still unidentified)
- Historical complaints
- Acute onset of dyspnea
- Evidence of thromboembolism
- Anorexia
- Vomiting
- Sudden death
- Sometimes, a stressful initiating event can be identified (anesthesia, surgery, fluids, steroids, change in household)
- PE findings
- Pulmonary edema: animal is severely dyspneic, increased lung sounds (crackles)
- Diastolic gallop (S4), soft murmur, or both
- +/- Hyperdynamic left apical impulse
- Lack of femoral pulses if thromboembolic disease present
- Some cats have left & right heart failure (pulmonary edema = most common)
- Pleural and/or pericardial effusion → muffled lung and/or heart sounds
- ECG: may be normal or have some combination of the following
- Conduction disturbances (esp. left axis shift or left anterior fascicular block [LAFB])
- LAFB = insensitive sign of concentric LV hypertrophy; can be seen in some normal cats
- LVE pattern and arrhythmias (VPCs)
- Atrial fibrillation if LA is enlarged
- Conduction disturbances (esp. left axis shift or left anterior fascicular block [LAFB])
- Thoracic radiographs
- Mild – moderate LVE w/ moderate – severe LAE
- Valentine-shaped heart on DV view
- Pulmonary venous distension & evidence of pulmonary edema (interstitial or fluffy alveolar pattern in cats)
- +/- Pleural effusion
- Longstanding HCM can → biventricular failure w/ marked pleural effusion, pericardial effusion, hepatomegaly, dilation of caudal vena cava
- Clinical pathology
- Azotemia & elevated liver enzymes = less frequent than with DCM
- Evaluate for hyperthyroidism & systemic hypertension, resulting from primary renal disease or other endocrine disorders
- Echo findings
- Hypertrophy of LV free wall and IVS present
- LA enlargement
- Fractional shortening is normal – increased
- Severe/advanced disease may → RVE or pericardial effusion
- +/- Systolic anterior motion of the mitral valve
- Doppler studies: turbulent flow in aortic outflow tract, mitral regurgitation
- Treatment
- If no clinical signs yet: aim to reduce heart’s ability to respond to stressful situations with an increase in HR → LV EDP
- Beta blockers (atenolol or propranolol) & calcium channel blockers (Diltiazem)
- Pulmonary edema: ACE inhibitors, furosemide, nitroglycerin (if severe dyspnea)
- Antithrombotics to prevent thromboembolism
- Cats diagnosed with HCM may have LV hypertrophy from a potentially reversible cause (i.e. hyperthyroidism, SHT, renal failure, acromegaly/endocrine diseases). So, explore the underlying cause for cardiac hypertrophy!
Restrictive Cardiomyopathy (RCM)
Group of cardiac diseases featuring LV endocardial abnormalities or restrictive myocardial filling abnormalities
- Primary RCM: ventricular filling is impaired
- Ventricle(s) is unable to distend appropriately in diastole, even in the presence of high filling pressures, due to restriction from endocardial thickening and endocardial/subendocardial fibrosis
- Decrease in force and extent that myofibers will shorten during systolic contraction
- The endocardium in some cats may appear normal upon examination, therefore, a myocardial restrictive disorder is suspected. The following disorders fulfill the criteria for a restrictive type of myocardial disorder:
- Endocardial Fibroelastosis: congenital anomaly.
- Thickening an opacity of the endocardium and a subendothelial layer of collagenous and elastic fibers
- Marked LV and LA dilation present at postmortem examination
- Cats (Burmese, Siamese), dogs, humans
- Clinical signs present at a young age (4 wk. – 4 mo.), with CHF and sudden death being a common sequela
- Excessive Left Ventricular Moderator Bands (accessory chords)
- Congenital anomaly in the cat: excessive moderator bands/accessory chordae tendinae w/in the LV (usually extending from LV free wall/papillary muscle → IVS)
- Recognized in young – middle aged cats
- Conduction abnormalities are possible (i.e. bundle branch block or AV block)
- Clinical signs are indistinguishable from other feline cardiomyopathies
- May accompany DCM, or may be a feline variation of normal in some cats
- Idiopathic Restrictive Cardiomyopathy
- RCM: endocardial or subendocardial thickening due to fibrosis or infiltrative disease
- Postmortem exam: focal areas of endocardial fibrosis in LV, papillary muscle fibrosis, multifocal myocardial necrosis and fibrosis, LAE
- Male predominance, middle aged – old cats
- Clinical signs are inconsistent, but present similarly to feline DCM or HCM cats. Potentially a higher incidence of thromboembolism, though
- ECG: LA and LVE patterns, arrhythmias possible
- Thoracic radiographs: can have dramatic LAE, also LVE, pericardial/pleural effusion, pulmonary edema, pulmonary venous distension
- Echo: LV and IVS hypertrophy possible, LA dilation. Fractional shortening is normal or slightly decreased. LV chamber may be reduced in size and areas of increased endocardial echo density (due to fibrosis) may be identified
- Treatment: similar to other cardiomyopathies
- Endocardial Fibroelastosis: congenital anomaly.
Unspecified Cardiomyopathy
Characteristics of more than one type of myocardial change are present (i.e. LV hypertrophy and dilation), or simply does not fulfill the criteria for DCM, HCM, HOCM, RCM or ARVC
- Typically accompanied by a restrictive filling pattern, as seen on spectral doppler studies if the patient has CHF
- Treatment based on the predominant clinical signs