Student Syllabus: Pathology of the Heart and Blood Vessels
Abbreviated Syllabus Download
PATHOLOGY OF THE HEART AND BLOOD VESSELS
Created by V’22 Cardio Group modified from Dr. Nicholas Robinson
Updated 3/27/20
Pathology of the Heart
Evaluation of the heart at necropsy
- Position
- Abnormal position
- Ectopia cordis – heart located outside of the chest
- Pericardial diaphragmatic hernia
- Compression
- Mediastinal mass
- Pyothorax
- Abnormal position
- Pericardium: Parietal layer, pericardial fluid, visceral layer
-
- Abnormal pericardium
- Red and thickened from edema and hyperemia
- Chronicity (>48-72 hours) to injury – mesothelial cells proliferation → shaggy, roughened appearance
- Pericardial effusion – excess pericardial fluid
- Causes: tear of proximal great vessels, neoplasia, myocardial rupture, coagulopathies
- Cardiac tamponade
- Affects right ventricle the most during diastole
- Abnormal pericardium
-
- Abnormalities of the great vessels
- Removal of the respiratory tract and heart en bloc
- Cardiac disease often affects the lungs and vice versa
- Trace pulmonary arteries
- Look for evidence of thrombosis and/or endoarteritis
- Evaluate cardiac chambers and valves by “following the flow”
- Examine internal and external features/lesions
- Endothelium: lines chambers of the heart, normally thin and almost invisible
- Subendocardial tissue: composed of fibroblasts, nerves, collagen, veins, and conduction system
- Endocardial thickening
- Result of fibrosis from a number of pathological conditions:
- scarring from “jet lesions”
- restrictive cardiomyopathy
- fibroelastosis
- Valvular structure
- Dysplasia
- AV valve most commonly affected
- Focal or diffuse thickened with fibrosis and abnormal, fibrotic, and shortened chordae attachments
- Some valve directly fused to the ventricular wall
- Stenosis
- Lesions affect the pulmonary valve more often, less in the aortic valve
- Stenosis may be in the valve or supra/subvalvular
- Rupture chordae → rapid valvular dysfunction and regurgitation
- Myxomatous valvular degeneration (Endocardiosis)
- The most common valvular lesion in dogs, often incidental finding in old dogs
- Grossly: free edges of valves thickened by 1-2 mm, smooth, shiny opaque white nodules
- Histologically: valve stroma is expanded by myxomatous materials
- May see concurrent chordae thickening and rupture
- in some small dogs → severe changes → marked deformation of the valves → valvular incompetency → secondary atrial enlargement and endocardial fibrosis
- Valvular endocarditis (vegetation)
- Fibrinous, yellow to tan to red irregular, roughened deposits on the valves
- Usually bacterial in origin → large number of pathogens associated with this lesion
- Histologically: vegetations are composed of fibrin, blood, inflammatory cells, and colonies of bacteria
- Inflammation can spread down the chordae → rupture
- Vegetations can break off → embolize into myocardium and lung
- Dysplasia
- Result of fibrosis from a number of pathological conditions:
- Heart is weighed after blood is removed and the great vessels trimmed off
- Cardiac diseases typically result in cardiomegaly rather than microcardia
- Normal heart weight in dogs: < 1% of body weight. Some dog breed (i.e. greyhound) have a higher normal heart:body weight ratio
- Obese animals → heart weight in the high normal heart to body weight ratio range should be viewed with suspicion for cardiomegaly
- Cats: absolute weight of the heart → useful indicator of disease
- Cat hearts should weigh less than 18-20 grams.
Reaction of the heart to altered hemodynamics
- Increased preload
- Increase blood volume → increased preload
- Acutely: stretching and dilation of the ventricle
- Accommodate end diastolic volume
- Increased pressure
- If preload persists → ventricle responds by laying sarcomeres in series
- “eccentric hypertrophy”
- Large volume chamber
- When limit is hit → congestive heart failure
- Altered conduction
- Perfusion to the area
- Morphologic changes to valvular apposition
- Increased afterload
- Increased resistance to the ejection of blood
- More force needed to empty the ventricles → sarcomere laid in parallel
- “concentric hypertrophy”
- Thickened ventricular wall
- Acquired disorder (e.g. hypertension)
- Marked hypertrophy → Increased perfusion distance from capillaries to the cells (capillaries do not grow with the cells)
- Hypoxia → abnormal conduction and dysrhythmias
- Congenital cases (e.g. stenosis)
- +/- Less severe hypertrophy
- Cardiac hyperplasia with some matching capillary growth
Myocardial Pathology
- Growth Disturbances
- Myocardial hypertrophy → increased muscle mass due to increase size of myocytes
- Compensatory response to increased workload/stimulation (reversible if stimulation removed)
- Primary Idiopathic Hypertrophic Cardiomyopathy → irreversible
- 2 gross anatomic forms
- Eccentric: enlarged ventricular chambers with thin-normal walls, result of increased blood volume load (e.g. valvular insufficiency or septal defect)
- Concentric: small ventricular chambers with thick walls, result of increased pressure load (e.g. valvular stenosis, systemic hypertension, pulmonary disease)
- 3 stages of hypertrophy
- Initiation
- Stable hyperfunction
- Dysfunction
- Right ventricular concentric hypertrophy
- Dirofilariasis → pulmonary hypertension
- Pulmonic stenosis in dogs
- Pulmonary hypertension from hypoxia in high altitude (cattle)
- Chronic alveolar emphysema (horses with heaves)
- Left ventricular concentric hypertrophy
- Subaortic stenosis
- Hyperthyroidism
- Systemic hypertension
- Biventricular hypertrophy
- Idiopathic Cardiomyopathy
- Some congenital anomalies
- End stages of heart disease due to other causes
- Myocardial hypertrophy → increased muscle mass due to increase size of myocytes
- Infiltration
- Fatty infiltration → adipocytes in myocardium → separate cardiomyocytes
- Obese animals, not associated with dysfunction
- Microscopic feature of arrhythmogenic right ventricular cardiomyopathy in certain dog breeds (e.g. Boxers)
- Fatty infiltration → adipocytes in myocardium → separate cardiomyocytes
- Degeneration
- Fatty degeneration
- Lipid droplets in the sarcoplasm
- Light microscopy → clear vacuoles, special stains to confirm the lipid
- Gross exam: heart may be pale pink to tan
- Occurs with severe anemia, copper deficiency, other systemic disorders
- Hydropic degeneration
- Vacuolated sarcoplasm, do not stain for lipid
- Classically occurs with anthracyclines
- Lipofuscinosis
- Accumulation of intralysosomal oxidized lipid residues
- Light microscopy → yellow brown granules near the cardiomyocyte nuclei
- Gross exam: heart may be brown or golden brown
- Age-related change
- Can occur in starvation or hereditary in Ayrshire cattle
-
- Myofibrillar degeneration
- Disruption of sarcoplasm → loss of cross striations and pale pink cytoplasm
- Classically seen with furazolidone toxicity in birds
- Potassium deficiency in rats
- Acute myocyte injury and many other toxicities
-
- Fatty degeneration
- Necrosis & Mineralization
- Necrosis → result from many types of injury
- Nutritional deficiency, toxin exposure, ischemia, metabolic disorder, physical injury, etc.
- Examples: ionophore toxicity (equine), vitamin E-selenium imbalance (neonates/ juveniles), anthracycline toxicity (dogs), gossypol toxicosis (pigs)
- Necrotic myocardium
- Gross exam: pale tan to white, sometimes gritty (rapid dystrophic mineralization)
- Microscopic: Swollen hypereosinophilic fibers with shrunken nuclei and basophilic cytoplasmic granules
- With chronicity, dead myofibrils are removed, myocytes may regenerate, and fibrosis can occur
-
- Mineralization → often occurs in conjunction with necrosis → calcium released from damaged sarcoplasmic reticulum
- Prominent in hereditary calcinosis (mice), vitamin D toxicosis, spontaneous in aged rats and guinea pigs
-
- Prominent in hereditary calcinosis (mice), vitamin D toxicosis, spontaneous in aged rats and guinea pigs
- Mineralized myocardium
- Gross exam: gritty, white
- Microscopic exam: basophilic, angular
-
- Necrosis → result from many types of injury
- Cardiomyopathies
- Primary cardiomyopathies (Idiopathic) → underlying causes are largely unknown
- Idiopathic Hypertrophic Cardiomyopathy (HCM)
- Increased left ventricular +/- interventricular thickness (concentric hypertrophy), most common in cats
- Gross: thick ventricle wall, small lumen. Weight > 20g
- Microscopic: none to enlarged myocytes and fiber disarray
- Pathophysiology: myocytes work harder and enlarge → hypertrophy reduces the compliance and diastolic function → impairs ventricular filling.
- Obstruction of left ventricular outflow during systole can be seen → forces generated by the narrowed outflow tract by septal hypertrophy → anterior motion of mitral valve leaflet
- Valvular displacement → mitral regurgitation → enlarge atria → blood stasis + inappropriate activation of endothelium → atrial thrombosis
- Associated genetic defect → myosin binding protein C gene mutation
- Idiopathic Dilated Cardiomyopathy (DCM)
- Ventricular and atrial dilation with ventricular hypertrophy (eccentric). Dogs, cats, cattle, poultry. Hypertrophy may not be obvious
- Subcategory: arrhythmogenic right ventricular cardiomyopathy
- Associated with ventricular tachycardia, most prominently in Boxer dogs
- Histopathology: right ventricle infiltrated by adipose and fibrous tissue
- Gross: big, heavy, flabby heart
- Microscopic: sometimes attenuated/wavy fibers
- Pathophysiology: decreased contractility and declining stroke volume → compensatory Frank Starling and neurohormonal mechanisms → myocytes elongate
- Long-term → compensatory mechanism not functional → myocyte degeneration → volume overload and failure
- Restrictive Cardiomyopathy (RCM)
- Primarily in cats, affecting left ventricle. Some evidence that RCM is preceded by endocarditis, but inciting cause is unknown
- Gross: thickened white left ventricular endocardium
- Microscopic: left ventricular endocardial and subendocardial fibrosis
- Pathophysiology: endocardial/subendocardial fibrosis + infiltrates → impair diastolic filling (ventricle more rigid than normal)
- Idiopathic Hypertrophic Cardiomyopathy (HCM)
- Secondary cardiomyopathies (known causes)
- Heritable
- DCM
- Human: mutation in several contractile protein and ion channel genes
- X-linked muscular dystrophy (Dogs), associated with subendocardial and interstitial fibrosis
- HCM
- Autosomal dominant in Maine Coon and American Shorthaired cats
- Mutations in sarcomeric proteins → mutation in the myosin binding protein C has been identified in heritable HCM in cats
- DCM
- Nutritional
- DCM: taurine deficiency
- Myocardial necrosis (due to deficiency): selenium-vit E imbalance, potassium, copper, thiamine, magnesium
- Toxic
- Myocardial necrosis: cobalt, catecholamines, ionophores, vit D and calcinogenic plants, blister beetles, rapeseed oil, T-2 mycotoxin
- Physical injury/Shock
- Myocardial necrosis: CNS lesions and trauma, GDV, electrical defibrillation, hemorrhagic shock
- Endocrine disorders → HCM: hyperthyroidism
- Infections
- Neoplastic
- Systemic hypertension in cats → HCM: spontaneous hypertension, renal disease, hyperthyroidism, diabetes mellitus, primary aldosterism
- Heritable
- Primary cardiomyopathies (Idiopathic) → underlying causes are largely unknown
Myocarditis → Inflammation of the myocardium, involve in a range of different inflammatory cells
Selected examples of etiologic agents
- Autoimmune: poorly documented, a hypersensitivity reaction (eosinophilic myocarditis) in cattle to plant toxin is known
- Parasitic:
- Sarcocytis spp Cysts: no immune reaction but may see eosinophils with degenerating cysts, multiple hosts
- Neospora caninum (dogs): myocarditis, myositis and encephalomyelitis
- Toxoplasma gondii (multiple hosts): myocarditis, systemic disease
- Larval tapeworms (multiple hosts): Taenia ovis, saginata, solium, and Echinococcus granulosum
- Trypanosom cruzi: Chagas disease, pyogranulomatous myocarditis
- Bacterial and fungal
- Generally → suppurative to necrotizing lesions
- Any pyogenic bacterium, more common ones include: Actinobacillus equuli, Clostridium chauvoei, Aspergillus terreus, Histophilus somni, Streptococcus spp.
- Viral
- Parvovirus and herpesvirus (puppies)
- Equine Herpesvirus – 1
- Foot and mouth disease → more necrotizing in young animals
- West Nile (raptors)
- Porcine circovirus/porcine parvovirus
- Encephalomyocarditis virus (swine, lab rodents)
- Plant toxins: cardiac glycosides
Myocardial necrosis
Selected examples of etiologic agents
- Vitamin E/Selenium responsive disease (pigs and ruminants)
- “mulberry heart” in pigs (2-4 months of age mainly)
- May see degeneration of arterioles in multiple organs
- “white muscle disease” in ruminants and horses → result of dystrophic mineralization of the myocardium
- Ionophore and gossypol toxicosis
- Ionophore in ruminant feed → control coccidian parasites and promote growth
- Mixing error → toxic doses added to feed
- Horses are very sensitive → peracute myocardial necrosis
- Gossypol → found in cottonseed meal, can be toxic in large quantities
- Ionophore in ruminant feed → control coccidian parasites and promote growth
- Doxorubicin toxicosis
- Anthracycline chemotherapeutic agent
- In dogs, can cause myocardial acute necrosis and chronic degeneration/necrosis via peroxidative injury and blockage of DNA, RNA, and protein synthesis
- Thromboembolic diseases
-
-
- Systemic inflammation, coagulopathies, vasculitis can result in cardiac necrosis
- Arteriosclerosis → rarely severe enough in animals to cause myocardial infarction
-
Myocardial neoplasia
- Primary tumors of myocardial cells → very rare
- Infiltrating tumors from other systems more commonly found
- Hemangiosarcoma in dogs
- One of the most common tumors
- Often found on the right auricular appendage
- Histologically: tumor composed of bizarre spindle cells → irregular vasculature
- Peripheral nerve sheath tumors in cattle
- Epicardial surface
- Most considered incidental findings
- Whirling spindle cells with supporting fibrous stroma
- Rhabdomyomas or muscular hamartomas
- Rare
- Most commonly found in pigs, have been reported in dogs, cattle, sheep
- B cell lymphoma
- Bovine leukosis virus in cattle
- Heart can be massively affected → little myocardium remaining with few or no clinical signs of heart failure
- Usual site → right atrium
- Chemodectoma (aortic body tumors)
- Heart base most often
- May be an incidental finding
- Hemangiosarcoma in dogs
Pathology of Blood Vessels
Normal Vasculature Composition:
- Arteries
- Intima: includes endothelial cells and the subintima
- Normally impermeable to large molecules
- Anti-inflammatory
- Resists leukocyte adhesion and thrombosis, promotes vasodilation
- Media: composed of elastin fibers, smooth muscle, and extracellular matrix
- Enables the contractile and elastic properties of the vessel
- Aorta/pulmonary artery:allows for stretch during systole and recoil during diastole
- Arterioles: more prominent muscle content allows for constriction/relaxation to regulate blood flow in response to circulating substances
- Can also produce extracellular matrix and inflammatory mediators
- Enables the contractile and elastic properties of the vessel
- Adventitia: contains nerves, lymphatics, blood vessels
- Intima: includes endothelial cells and the subintima
- Veins are very similar to arteries EXCEPT:
- Thinner subendothelial layer and muscular media
- Typically lack intrinsic vasomotor activity → blood flow is dependent on external compression of the skeletal muscle and one-way valves
Vasculature Reactions
- Intimal Thickening: non-contractile smooth muscle cells are recruited via endothelial signals into the intima, where they divide and produce ECM
- Usually a response to vessel injury or aging
- Even with normalization of the endothelial layer intimal thickening remains
- Altered Thrombotic Surfaces:
- Physiologic: normal endothelial activation with maintenance of antithrombotic properties and appropriate smooth muscle tone
- Pathologic: endothelial dysfunction as a result of detrimental stimuli (e.g. viral/bacterial infections, hypertension) or excessive physiologic stimuli (persistent hypoxia/acidosis, cytokines)
- Results in pro-thrombogenic surface and/or abnormal signaling to the underlying smooth muscle cells (altered vasoreactivity)
- Altered vascular reactivity and medial proliferation: smooth muscle can dilate or constrict vessels in response to physiologic/pathologic stimuli
- Arteriosclerosis: a common general reaction pattern to endothelial damage that results in a loss of elasticity and wall thickening
- Thickenings are grossly white and intimal surface may appear wrinkled
- Result of multiple causes (hypertension, calcification, plaques, etc) related to altered hemodynamics
- Hypertension: causes increased extracellular matrix deposition in vessels due to protein leakage across damaged endothelia (“hyaline arteriosclerosis”)
- Persistent Hypercalcemia/Inflammation/Aging: may cause arterial calcification (“medial sclerosis”)
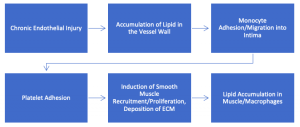
- Atherosclerosis: a type of arteriosclerosis that is uncommon in domestic animals (may occur in rabbits, chickens, parrots, non-human primates, and pigs)
- Regarded as a healing response to a chronic inflammatory condition that affects large elastic (aorta) and medium muscular (i.e. coronary, femoral) arteries
- Hallmark is yellow, irregular, and raised intimal “plaques” that protrude into the lumen and are often mineralized
- Composed of foamy cells of likely smooth muscle origin, monocytes/macrophages, and accumulations of lipid
- Plaques surfaces are highly thrombogenic and can cause ischemia
- Pressure to the underlying media can weaken the vessel and cause pathologic dilation/rupture
- Pathogenesis:
- Aneurysms: inherited/congenital or acquired focal abnormal dilation of the vessel wall
- Usually the result of vessel alternation in three ways:
-
-
- Congenital defects to connective tissue (e.g. Marfan syndrome)
- Increased collagen destruction/decreased collagen synthesis (e.g. protease activity in inflammatory conditions)
- Loss of smooth muscle and/or synthesis of non-elastic ECM (e.g. “cystic medial degeneration”)
-
-
- False aneurysm: a vessel bulge created by an extravasated focal hematoma forming in the wall between the tunica media and adventitia
Vascular Conditions in Veterinary Species
- Vasculitis: inflammation within the blood vessel, causing vessel wall damage
- Hallmarks: perivascular/vascular fibrin deposition, endothelial and stromal cell necrosis, and/or collagen degeneration
- NOTE: perivascular inflammatory cell presence is NOT sufficient to diagnose vasculitis!
- Can cause thrombosis and hemorrhage with downstream ischemia
- Some important causes of vasculitis include:
- Viral Disease (ex. FIP, equine arteritis virus, African horse sickness, African swine fever, hog cholera/classical swine fever)
- Autoimmune Disease (ex. polyarteritis nodosa, hypersensitivity vasculitides)
- Bacterial/Rickettsial Agents (ex. Rocky Mountain fever, heartwater, hepatic abscesses in cattle)
- Fungal Disease: (ex. mycotic ruminitis, guttural pouch mycosis)
- Parasitic: (ex. Strongylus vulgaris, schistosomiasis, Dirofilaria immitis)
- Hallmarks: perivascular/vascular fibrin deposition, endothelial and stromal cell necrosis, and/or collagen degeneration
- Arteriosclerosis:
- Systemic hypertension: can be a cause or effect of renal disease, or related to pheochromocytoma, diabetes mellitus, or hyperthyroidism (among others)
- Self-perpetuating (increased pressures lead to medial hypertrophy/hyalinization → decreased perfusion → more hypertension)
- Can also result in retinal degeneration, hemorrhage and detachment
- Pulmonary hypertension (PH): can be a cause or result of pulmonary arterial disease, or related to cardiac diseases (ex. left to right shunting) or medial proliferation secondary to arteritis
- Hypoxia → pulmonary arterial constriction/hypertrophy → hypertension
- Cattle and pigs can develop hypertensive heart failure at high altitudes
- Mineralization: dystrophic or metastatic types (ex. vitamin D toxicity, Johne’s disease in cattle, hypercalcemia)
- Uremia: endothelial damage causes fibrin leakage into the media and collagen degeneration within the wall
- Calcification may also occur from hypercalcemia/hyperphosphatemia
- Systemic hypertension: can be a cause or effect of renal disease, or related to pheochromocytoma, diabetes mellitus, or hyperthyroidism (among others)
- Aneurysmal conditions: often secondary to inflammation or hemodynamic changes (ex. Marfan syndrome, Copper deficiency in swine)
- Miscellaneous syndromes
- Cystic rete ovarii in cats, ovarian varicosities in horses, uterine artery rupture in horses: degenerative conditions of the reproductive vasculature that may result in infertility and/or hemoabdomen
- Aortic rupture in horses: uncommon but thought to be secondary to increased aortic pressure; hemorrhage can occur in the pericardium, myocardium or thoracic cavity
- Telangiectasia in the liver of cats/cattle: multifocal, small dilations of the hepatic sinusoids that present as blood filled plaques on the subcapsular surface
- Neoplastic conditions
- Endothelial cell tumors
- Hemangiomas/hamartomas: benign, proliferative conditions of endothelial cells often in the dermis/subcutis
- Present as a red-pink nodule or plaque with or without ulceration (may look like granulation tissue in horses)
- Complete excision is curative
- Hemangiosarcoma: often very aggressive, malignant endothelial tumors
- Cutaneous/peripheral soft tissue: less aggressive than visceral form
- Visceral: commonly seen in right auricle, spleen, liver, kidney, and retroperitoneum with widespread metastasis to the lung, body cavities and brain
- Clinical signs related to coagulation dysregulation from blood flowing/clotting in neoplastic vessels and/or hemoabdomen/hemothorax/hemopericardium
- Hemangiomas/hamartomas: benign, proliferative conditions of endothelial cells often in the dermis/subcutis
- Vascular wall tumors: benign/low-grade malignancies derived from smooth muscle of the wall (leiomyoangioma/angiosarcoma) or from supporting cells (hemangiopericytoma)
- Endothelial cell tumors
- Malformation conditions of the vasculature
- AV fistulas: small abnormal connections between arteries and veins that bypass the capillary bed; can be the result of congenital factors, trauma or inflammation
- Portosystemic shunts: abnormal placement of a vessel connecting the portal and system circulation