Interaction with MDM2
One of the genes that p53 can bind to encodes for the protein MDM2 [19]. MDM2, in turn, is a tri-functional protein that binds p53 with high affinity. By simply binding p53, MDM2 sequesters it, lowering its concentration in the nucleus and reducing its ability to act as a transcription factor. MDM2 also can export p53 from the nucleus into the cytosol, where it is effectively useless [20]. Finally, MDM2 can ubiquitinate p53, which targets p53 for degradation [21]. In normal cells, MDM2 prevents p53 from activating pathways that would kill the cell. However, if MDM2 is overexpressed when DNA damage occurs, p53 will be ineffective and replication can continue unchecked (Figure 1). MDM2 overexpression is common in many tumor cells.
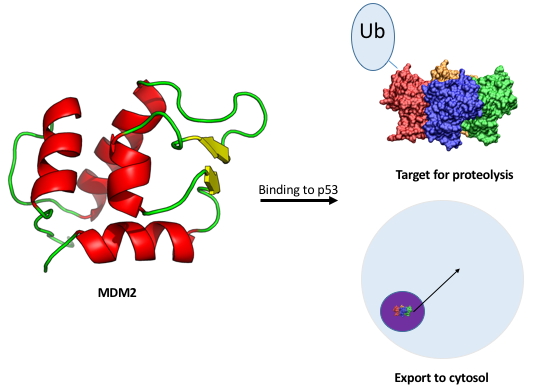
Figure 1: Schematic diagram showing possible effects of p53 binding to MDM2. At top, ubiquitination, marking the tumor suppressor for proteolysis. At bottom, export to cytosol. PDB ID: 1YCR, 5LGY [22,12].
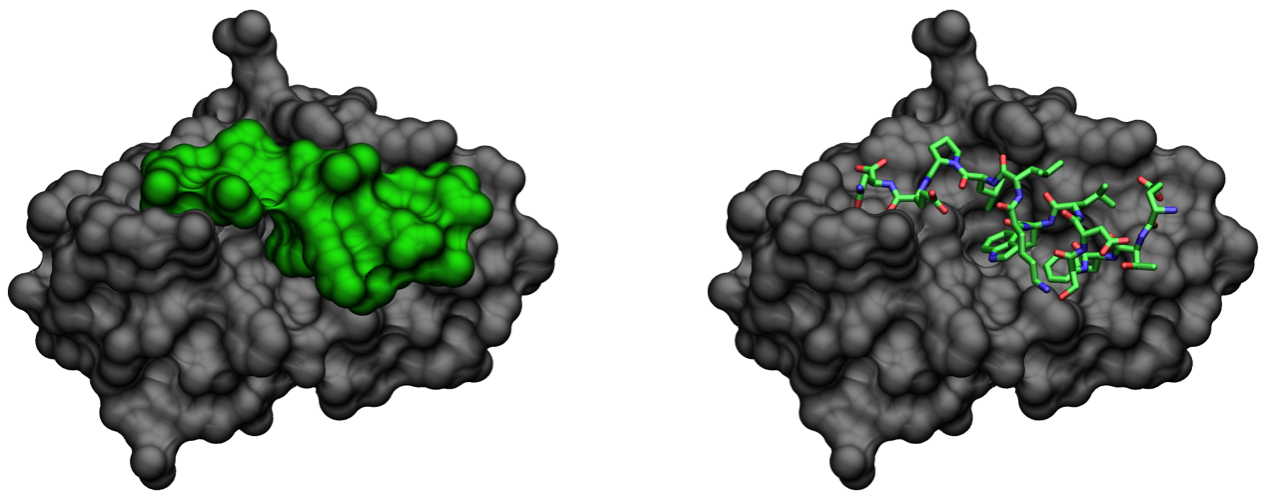
Figure 2: MDM2 (gray) in complex with a 14 residue fragment (15-29) of transactivation domain of the p53 tumor suppressor (lime) (PDB: 1YCR) [22]. Molecular surfaces calculated using MSMS [23].
A 14-residue fragment of p53 from its transactivation domain has been crystallized in complex with MDM2, indicating that the portion of p53 that actually binds MDM2 is quite small (Figure 2) [22]. Given the importance of this interaction in cancer, developing selective high affinity binders for MDM2 is a highly active area of investigation in drug design [20].
MDMX, a related oncogene product
While the focus of this project is primarily the inhibition of the interaction between p53 and MDM2, it is important to note the existence of structurally homologous gene product, MDMX, also implicated in cancers. MDMX binds to p53 similarly to MDM2 at the transactivation domain, and can prevent p53’s activation. The main difference between the two is that MDMX itself is not an ubiquitin ligase and cannot shuttle p53 from the nucleus to the cytosol. Nonetheless, its role is important in cancer because it does still serve to reduce tumor suppressor activity [31]. The binding cleft is highly similar to that of MDM2, so it is possible that drug design targeting MDM2 may secondarily target MDMX [32].
Recent Comments