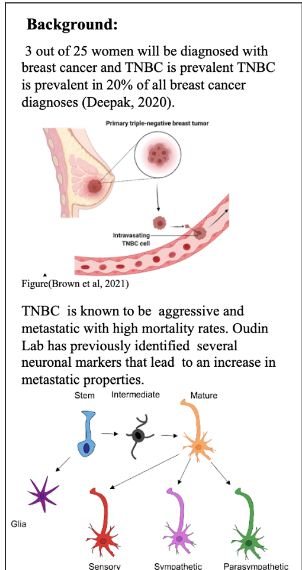
Breast cancer is caused by genetic mutations in epithelial cells of the breast. These mutations dysregulate the proliferative or apoptotic signaling pathways which create aggregates of harmful nonfunctional cells called tumors. The tumors disrupt normal cell function and can be fatal if left untreated. Classification of breast cancers is based on the surface receptors that the cell line expresses. Surface receptors tend to make the cancer more aggressive, but they can also serve as therapeutic targets. There are four molecular subtypes of breast cancer: luminal A, luminal B, HER2+, and triple-negative. This study specifically uses TNBC, which does not express any surface receptors.
Cancer cells spread in metastasis throughout the body via the lymphatic system, the bloodstream, or through perineural invasion, a process dependent on cell migration, which is the leading cause of death in cancer patients10. Metastatic triple negative breast cancer is fatal 11. Chemotherapy remains the only FDA-approved treatment for TNBC; however, it has significant negative side effects including hair loss, appetite loss, and anemia. This treatment does not prevent metastasis, as roughly 30% of early-stage breast cancers become metastatic regardless of chemotherapy12. Research is currently being performed to seek out potential targets for new therapies for TNBC13,14.
A recent study by Yang et al found upregulated neuronal genes, TUBB3 and Tau, in small cell lung cancer leading to elongated cell shape and saltatory movement, increasing metastatic potential15. Complementary, publicly available transcriptome data of triple negative breast cancer cells has shown upregulation of neuronal genes. Thanh Le et al. did a mRNA study on patient samples with inductive ductal carcinoma, the most common type of breast cancer, which showed increased mRNA expression of neuronal genes TUBB3, CNTNAP2, SEMA4D and MAPT in human invasive ductal carcinoma versus normal breast tissue (Figure 1A)6,8.

Figure 1: A) mRNA expression of neuronal genes TUBB3, CNTNAP2, SEMA4D and MAPT in human invasive ductal carcinoma versus normal breast tissue, each column represents a patient6
Two neuronal genes, TUBB3 and MAPT, have been identified as highly expressed in breast cancer and biomarkers for poor prognosis. TUBB3 has been previously considered to promote microtubule dynamics and MAPT to have a stabilizing effect on microtubules16,17. MAPT and TUBB3 therefore have opposite roles on microtubule dynamics but at the same time induce similar effects in cell morphology and migration8. Clinical data indicates a correlation between resistance to taxane-based chemotherapies and overexpression of MAPT and TUBB31,2. Taxane-based chemotherapies, like paclitaxel, are able to induce cell death by stabilizing microtubules. Since TUBB3 and MAPT play roles in microtubule dynamics, they likely impact the effectiveness of these types of chemotherapies. Doxorubicin, on the other hand, induces apoptosis through DNA damage and it does not affect microtubule dynamics.
Preliminary research in the Oudin Lab revealed that knockdown of TUBB3 and MAPT in MDA-MB-231 breast cancer cell line led to increased cell speed, saltatory movements, and migration, indicating their potential role in metastasis8. Investigating the effects of gene knockdown on TNBC cells may reveal new therapeutic avenues for exploiting higher chemotherapy sensitivity while limiting metastasis potential and identifying these genes as biomarkers for breast cancer.