RESULTS:
Optimal concentration and incubation time for siRNA knockdown found
To create a platform to study the effects of these genes, siRNA knockdown was optimized and compared to the previous method of CRISPR knockdown. To optimize this method of knockdown, three concentrations of siRNA will be tested: 5μM, 2.5μM, and 1.25μM. These concentrations were chosen based on the recommendations from Horizon Discovery Biosciences, where the siRNA came from. Incubation times of 24 hours and 72 hours were tested for MAPT knockdown, and 24 hours and 48 hours were tested for TUBB3 knockdown. 48 hours was assessed for TUBB3 knockdown since the cells appeared to be too confluent to wait to be assessed at the 72 hour time point. As stated before, the metric for successful knockdown is at least 70% knockdown.

Figure 2: Comparison of CRISPR and siRNA knockdown methods. A) CRISPR knockdown quantification for MAPT using qpcr (left) and for TUBB3 using Western blot (right). B) SiRNA knockdown quantification for MAPT using qpcr (left) and for TUBB3 using immunostaining (right). C) 2D migration speed of 231-Scr control and CRISPR MAPT knockdown cells (left) and CRISPR TUBB3 knockdown cells (right). D) 2D migration speed of 231-Scr control and siRNA MAPT and TUBB3 knockdown cells. Significance was determined by one-way ANOVA (***p<0.001, ****p<0.0001).
Previously in the lab, MAPT and TUBB3 had successful knockdowns. MAPT mRNA expression level was about 75% of the MDA-MB-231 control cells and TUBB3 expression level dropped to approximately 10 and 30% of the 231-Cas9 control cells (Figure 2A). MAPT siRNA knockdown results show that there is almost 100% knockdown after incubation with siRNA for 24 hours at all concentrations (Figure 2B). Data that tested the siRNA concentration of 2.4μM could not be obtained due to a reading error by the thermal cycler. At the 72 hour time-point, there is about 60-70% MAPT knockdown across all concentrations (Figure 2B). TUBB3 siRNA knockdown results show about 80-95% knockdown after incubation with siRNA for 24 hours across the different concentrations. After incubation with the siRNA for 48 hours, there was about 25% knockdown at 5μM siRNA. There was no knockdown of TUBB3 at the two lower concentrations of siRNA (Figure 2B).
To compare knockdown methods, migration assays were carried out on both sets of cells (Figure 2C and Figure 2D). MAPT and TUBB3 CRISPR knockdown cells moved significantly faster than control cells. However, no differences in cell speed were seen when the siRNA knockdown platform was used.
To test the effect of siRNA knockdown in the MDA-MB-231 cell line, we additionally performed an adhesion assay to assess the effects of siRNA knockdown on cell morphology. However, we encountered challenges in accurately analyzing the cells due to excessive confluency, as seen in Appendix 1. Consequently, we were unable to perform the adhesion assay analysis for these cells.
Due to siRNA knockdown being an inefficient platform to study these genes, we proceeded to investigate the effects of knockdown only using the CRISPR cells in the following experiments.
Gene knockdown leads to susceptibility to chemotherapy at 48 hour time point
In order to assess the effectiveness of chemotherapy after gene knockdown, we performed Presto Blue viability assays after administering chemotherapy to CRISPR knockdown cells. Viability was assessed 48 hours after chemotherapy exposure. This time point was optimized after preliminary tests were performed at various time points.
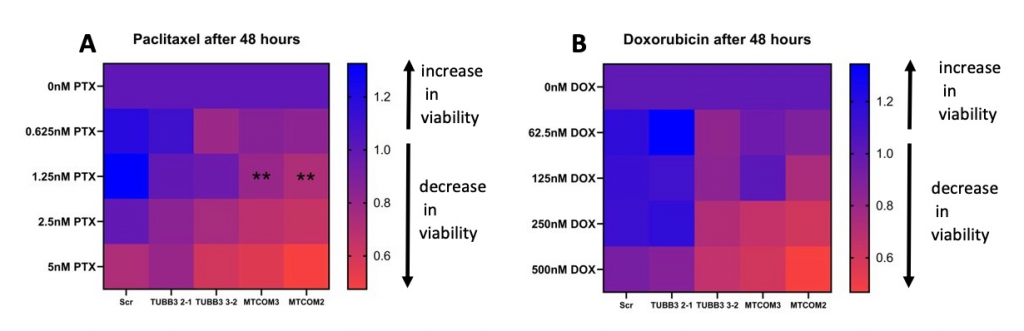
Figure 3: MAPT knockdown sensitizes MDA-MB-231 cells to taxane chemotherapy. A) Day3/Day1 viability fold change of CRISPR MAPT and TUBB3 knockdown cells after paclitaxel treatment and B) doxorubicin treatment. Each group (MAPT and TUBB3 knockdown) was compared to control cells (Scramble-Scr). Significance was determined by two-way ANOVA (**p<0.01).
Upon exposure to paclitaxel, both replicates of MAPT knockdown demonstrated a statistically significant decrease in viability compared to the control after 48 hours. TUBB3 knockdown cells had no significant difference in viability. Knockdown did not affect viability of the cells after doxorubicin exposure.
Gene knockdown leads to morphological changes due to chemotherapy exposure
Besides effects of chemotherapy on viability, effects on morphology should be analyzed as well. Morphology indicates the metastatic potential of the cell. To assess this, the cells were incubated with pacitaxel and doxorubicin and adhesion assays were performed.
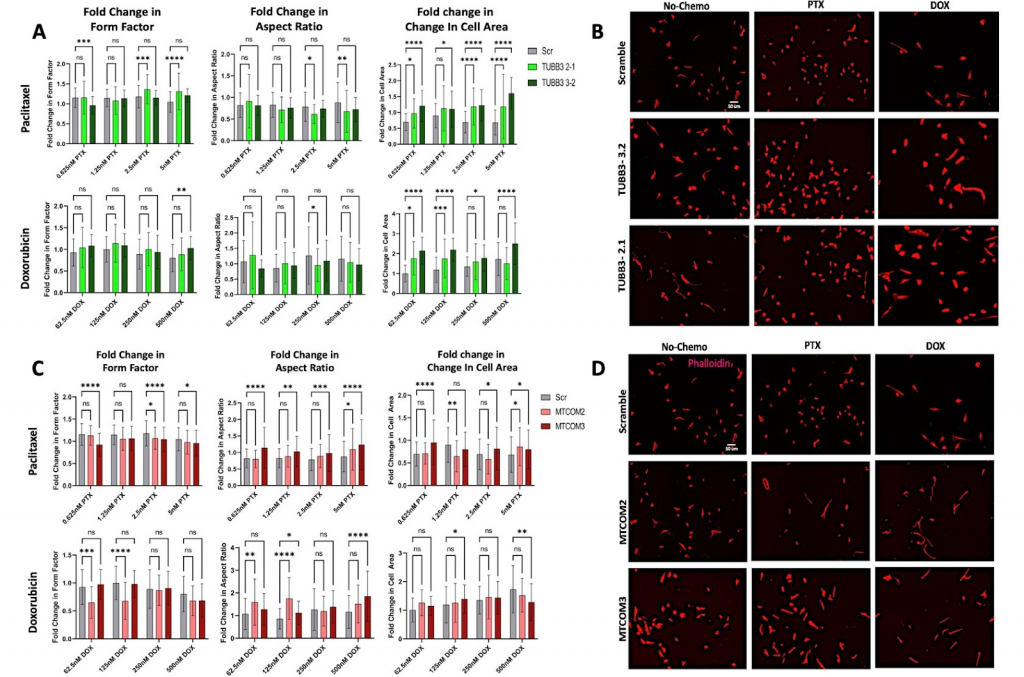
Figure 4: Effects of chemotherapy treatment on cell morphology of TUBB3 and MAPT CRISPR knockdown cells. A) Graphs show fold change in form factor, aspect ratio, and cell area of TUBB3 knockdown cells after paclitaxel and doxorubicin treatment. B) Images obtained of TUBB3 knockdown cells with no treatment, paclitaxel treatment, and doxorubicin treatment. C) Graphs show fold change in form factor, aspect ratio, and cell area of MAPT knockdown cells after paclitaxel and doxorubicin treatment. D) Images obtained of MAPT knockdown cells with no treatment, paclitaxel treatment, and doxorubicin treatment. Significance was determined by two-way ANOVA (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
One replicate of TUBB3 knockdown had significantly smaller average aspect ratio when subjected to one concentration of doxorubicin (250nM) and to two concentrations of paclitaxel (2.5nM and 5nM). The results also indicate that chemotherapy leads to a larger form factor for one concentration of doxorubicin (500nM) and three concentrations of paclitaxel (0.625nM, 2.5nM, and 5nM). Lastly, both replicates of TUBB3 knockdown had larger cell areas across all concentrations of both chemotherapies. Overall, the trends seen were that TUBB3 knockdown cells had slightly smaller aspect ratios, larger form factors, and larger cell areas when subjected to chemotherapy.
Knockdown of MAPT led to larger aspect ratios of the cells for ¾ of the concentrations of doxorubicin, and for all four concentrations of paclitaxel. In addition, it leads to smaller form factors for ½ of the concentrations of doxorubicin, and for ¾ of the concentrations of paclitaxel. In terms of changes in cell area after doxorubicin treatment, one replicate of MAPT knockdown had an increase in cell area at 125nM, and it had a decrease in area at 500nM. MAPT knockdown cells had an increase in cell area after being subjected to 0.625nM, 2.5nM, and 5nM of paclitaxel. However, it had a decrease in cell area when subjected to 1.25nM of paclitaxel. Overall, these results suggest that MAPT knockdown leads to larger aspect ratios and smaller form factors when exposed to chemotherapy. No conclusions can be drawn about fold changes in cell area due to contradicting data.
DISCUSSION:
Our preliminary results suggest that using CRISPR for knockdown is a more effective platform to study these genes in triple-negative breast cancer cells. CRISPR knockdown led to sufficient, permanent knockdown of MAPT and TUBB3, which led to significant differences in the cells themselves (Figure 1A). SiRNA knockdown was sufficient for the first 24 hours, but the cells started to proliferate quicker than normal, and the genes quickly became un-silenced. For TUBB3 specifically, the gene was already fully expressed again only 72 hours after knockdown (Figure 1B). This is an issue, since many assays that evaluate metastatic potential can take up to four days. To see if these two knockdown methods induced consistent effects in the cells, migration and adhesion assays were performed on both. Knockdown of MAPT and TUBB3 using CRISPR led to faster cell speed (Figure 1C). Faster cell speed indicates it is more likely for the cells to migrate and metastasize. No significant differences in cell speed were seen when siRNA was used for knockdown (Figure 1D). This suggests siRNA knockdown is not a sufficient method to analyze knockdown effects due to the heterogeneity among the cells and the quick regeneration of gene expression. Adhesion assay data could not be obtained after siRNA knockdown due to excessive proliferation. This can be seen since siRNA controls vital processes like cell proliferation, also rendering it a less desired method of knockdown. These findings support our belief that CRISPR may be a more effective method for analyzing neuronal genes in TNBC. This study emphasizes the importance of carefully considering appropriate experimental methods to ensure accurate and meaningful results. SiRNA knockdown led to heterogeneity in our data that could not be analyzed, so CRISPR cells were used in the following experiments.
The rest of our experiments involved characterization of the effects of CRISPR knockdown on metastatic potential and chemotherapy sensitivity. We first explored the effects of knockdown on chemotherapy sensitivity through Presto blue viability assays (Figure 2). The motive behind this exploration is that clinical data indicates a correlation between resistance to taxane based chemotherapies and an overexpression of MAPT and TUBB32. This is likely through their mechanism of action: taxane-based chemotherapies are able to induce cell death by stabilizing microtubules. Since TUBB3 and MAPT play roles in microtubule dynamics, they likely have an impact on the effectiveness of taxane-based chemotherapies like paclitaxel. Another chemotherapy, doxorubicin, kills cells by inducing DNA damage. This drug will also be tested as a control to see if knockdown affects only chemotherapies that target microtubules.
Our results indicate that MAPT knockdown sensitizes cells to 1.25nM paclitaxel, and this was consistent across both replicates. TUBB3 knockdown appeared to have no significant effect on sensitivity to chemotherapy. Knockdown had no significant effect on sensitivity to doxorubicin. These findings are consistent with a previous study that found overexpression of these genes is correlated with taxane-based chemotherapy resistance. Overall, our data suggests that upregulation of MAPT causes resistance to taxane-based chemotherapies.
We also explored the effects of knockdown on morphological changes due to chemotherapy exposure. In addition to chemotherapy playing a role in cell viability, chemotherapy can induce morphological changes that allow the cells to migrate, thus metastasize, more invasively. It is important to not only consider the effects on viability, but also the changes in metastatic potential itself. Many studies have shown that cell morphology is indicative of invasive capacity. Three parameters were assessed: form factor, aspect ratio, and cell area. TUBB3 knockdown cells gained larger form factors and larger cell areas after both chemotherapies were administered, both of which are correlated with higher invasive capacity (Figure 4A). So, our study has indicated that TUBB3 knockdown does not sensitize cells to chemotherapy, and it appears the chemotherapy actually may make the cells more susceptible to metastasize according to their morphological changes.
MAPT knockdown cells had significantly smaller form factors after they are subjected to chemotherapies. This suggests that they have decreased invasive capacity after chemotherapy is administered. Therefore, MAPT knockdown leads to higher sensitivity to taxane-based chemotherapy, and the cells themselves become less likely to metastasize. MAPT knockdown also leads to faster cell speeds, but knockdown in combination with chemotherapy appears to kill the cells while minimizing the risk of metastasis.
These preliminary results suggest that there’s correlation between neuronal genes MAPT and TUBB3 and the effectiveness of chemotherapy. If the differences seen in the above figures are true, MAPT may serve as a potential therapeutic target for TNBC. Overall, this data may also motivate the progression of further research in the neuronal identity of TNBC. We were able to design and optimize the conditions of the best platform to study these genes: CRISPR. The platform we designed will be utilized by other researchers to continue to study these genes and further characterize this subtype of triple-negative breast cancer. Characterization is important, because many patients with mild tumors can receive chemotherapy unnecessarily, which subjects them to significant side effects and a decrease in quality of life. By performing genetic screening, the severity of the tumor can be determined by looking at the relative expression levels of genes like MAPT and TUBB3. Since MAPT and TUBB3 are correlated with higher malignancy1,16, a physician may be more inclined to start a chemotherapy regimen. Conversely, if a tumor lacks malignant biomarkers, the chemotherapy treatment can be deemed unnecessary. This gives physicians more comprehensive information to improve their clinical decisions and tailor their treatment more specifically to each individual’s illness. Before this can happen, the physician’s decision has to be contextualized to understand the complex effects of neuronal genes on TNBC cell behavior. Knowing how patients with upregulated TUBB3 and MAPT respond to different chemotherapies, how likely the cancer is to recur, and how these genes affect the pathways involved with metastasis will allow physicians to treat their patients with the best treatment and care. These genes themselves may be the targets of new and more effective therapeutic treatments. So, our findings suggest that MAPT plays a critical role in chemotherapy sensitivity and metastatic potential. The platform we designed will allow the cancer community to further this understanding and eventually improve patient outcomes with this subtype.