Electrical Batteries for Renewable Energy
Abstract
The lead acid battery is the most used in industry. It’s advantageous to use because of its low cost. Modern renewable energy systems need batteries to operate properly and implement different charging techniques. Solar panels can only produce electricity during daylight hours and use batteries to store electricity for later use. The effectiveness of the system is limited to the batteries ability to store and release the energy. Engineers are looking into better ways to utilize the batteries in the system. The wind turning a turbine generator changes speeds at almost every instant. Batteries are used to stabilize the inconsistent energy surges to be useful. The system is only an effective means of producing consumable power when the batteries can stabilize the systems energy effectively. New battery technology is being developed to replace the lead acid technology and make systems more cost effective.
Background
Introduction
The definition of a battery is a device that converts chemical energy directly to electricity. (Al-Sheikh, Moubayed, 2012) The most common types used today are the lithium ion and the lead acid battery. The lithium ion is mainly used in portable electronics including a cell phone or laptop because they can store a very large amount of electricity in a very small battery. However, when produced in bigger sizes they become very expensive because the materials and complexity of producing the batteries. The lead acid battery is mainly used in these higher power applications. It is an integral part of the overnight storage of solar energy. Specific systems are built around these batteries so they can supply electricity at all times. Most renewable energy systems today use batteries to preform two different essential operations. One being the storage of the energy produced and the other being a connection to smooth the energy being produced. These implementations are seen in wind and solar energy. These systems still use lead acid batteries, which now are considered old technology. Currently, longer lasting batteries are being developed to replace the lead acid in these systems to cut costs.
Table 1
Major Battery Types for Renewable Energy Sources.
| Battery Type | Advantages | Disadvantages | Common Uses |
|---|---|---|---|
| Lead Acid | -Low Cost-Mass Produced | -Heavy-Harmful Chemicals
-High maintenance -Short Life |
-Solar Panel Systems-Wind power Systems
-Standby Applications |
| Lithium Ion | -Small Size-Low Weight
-High Energy Capacity -Long Life |
-Expensive-Difficult to Produce | -Small Electronics |
| Ultracapacitor | -High Power-Long Life
-Easy Storage -Quick Charging |
-Expensive-Difficult to Produce | -None yet |
Lifespan
Inside the lead acid battery, sulfuric acid chemically reacts with a specific type of lead to create electricity. (Al-Sheikh, Moubayed, 2012) The reverse process charges the battery and creates the original acid and lead. The production of these types of batteries is very easy and they are mass produced around the world, making the lead acid battery the most cost effective battery for large power applications.
Since the lead acid battery is the most cost effective battery to use in these systems, lots of research has gone into understanding the processes inside. The average lifespan of a lead acid battery is 1000-2000 cycles at 70% discharge. (Al-Sheikh, Moubayed, 2012) The cycles are the amount of times the battery is charged up and then discharged for use. The 70% discharge means that the battery is depleted from 100% of its electrical power to 30% before it was replenished. As a result of the chemical process inside the lead acid battery the lifespan of the battery is greatly reduced if the battery is run fully out of charge. When fully discharged some of the chemical liquids in the battery are broken down to their maximum potential and the process becomes irreversible. This is called stratification. In order to maintain the life of the battery, most systems only discharge the battery to a certain percentage before turning the system off. It is also detrimental to store the battery for a period of time on a low charge. When the battery is depleted down to 30%, it should be recharged very soon or sulfation will occur. When sulfation occurs the chemical deposits made by discharging harden over time and become unreactive. (Glavin, Hurley 2006) These deposits are no longer useful in the battery and reduce the power it can produce exponentially.
Significantly overcharging also can reduce the batteries lifespan. The process known as gassing can occur if the battery is left charging past its maximum point. (Glavin, Hurley 2006) When the sulfuric acid and lead revert their original states, and the battery is fully charged, the extra electricity reacts with the water inside the battery causing it to evaporate and leave the battery through its vents. This makes the acid less effective at reacting and therefore making the battery less powerful.
Charging Techniques
Specific charging methods must be implemented to the system to maximize the batteries lifespan. One of the most common techniques used to charge standalone batteries involves three different parts. (Glavin, Hurley 2006) The first is a constant charge until the battery reaches 95% of its maximum. Then the second stage is a float charge, where the battery is charged to its fullest then disconnected and used until it hits the 95% threshold. Then it is charged again and let back down to the threshold. This can be seen in the figure below. By doing this, the battery will not overcharge and minimal gassing will occur. This floating is done until the battery is put to use and discharged. The third stage of charging involves float charging at a lower threshold to equalize the cells and should be done periodically. These charging techniques are implemented in almost all systems that the lead acid battery is placed into.
Figure 1
Float charging method during normal operation of a battery.
Solar Panels
Solar panels or photovoltaic systems are a very popular and an up and coming renewable energy. Large silicon based tiles are placed in an unobstructed area to take in sunlight. The tiles transform this sunlight into electricity. Batteries are specifically useful for these photovoltaic systems that are off “the grid”. The grid refers to the interconnected network of houses or buildings to a power plant. If a system is on the grid it is connected to that network and use power made elsewhere. When systems are referred as off the grid is the total disconnection from a power plant. All of the electricity that can be used in the system must be produced on its own. In this case, the off-grid energy is produced by solar panels. These solar panels only produce usable electricity during daylight hours. In order for the electricity to be used at a later time it must be stored in a battery(Glavin, Hurley 2006).
In the off grid photovoltaic system the solar tiles act as the primary source of energy while the battery acts as a backup source. When solar electricity is available it is used but when it is not the battery power is used. (Parsekar, Kishore 2014) With this configuration, not all electricity harnessed by the tiles can be used because of the limits of the batteries. In order for these systems to be cost effective, lead acid batteries are used. The size and amount of batteries used are dependent on the electricity that is used within the system.
When configuring the systems, the batteries are maximized because they have the shortest lifespan and are one of the most expensive components. As described above, certain charging techniques are used to significantly increase the lifespan of the batteries used. In order to reduce the detrimental effects of stratification, the batteries cannot be depleted of all their stored electricity. They can only be discharged to a certain extent and therefore making the system to unable to work at its maximum potential. When the batteries are discharged they must be recharged as quickly as possible in order to avoid sulfation which also decreases the life of the battery.
The photovoltaic system must also implement the technique of float charging the batteries in order to avoid gassing. As described above the float technique does not guarantee that the batteries will be charged to their full potential when sunlight is removed from the system. The float technique only guarantees the maximum charge is within a certain threshold. These limits placed on the system by the different charging techniques do not allow it to operate at its maximum potential, but are implemented done because they decrease the overall cost of the system.
With batteries being one of the most expensive and most replaced parts in a photovoltaic system they are the limiting factor for the potential of the systems as well as the financial feasibility. Lead acid batteries are most commonly used because they are mass produced, but they are not the ideal battery. The entire system must be set up to maximize the battery life, therefore limiting other parts. With replacement every 1000-2000 cycles, the cost to maintain the system quickly adds up and in some cases becomes too expensive.
Wind Energy
Wind energy is also a very popular renewable energy that requires batteries to operate. Wind energy is the fastest growing renewable energy technology in the world. (Seoud, Jatskevich 2008) Wind energy uses a large propeller to rotate a turbine that creates electricity. Massive amounts of these turbines are set up in windy areas and make up wind farms. Batteries are useful for wind energy systems that are connected as well as disconnected to the grid. This is because batteries can be used in two different applications within the wind energy system. Like the photovoltaic system, batteries can be used for off grid storage of electricity during non-windy times. The wind however, cannot be predicted as easy as the sun so applying different charging techniques does not really help the lifespan of the batteries used.
The second application of batteries within wind energy systems that are both on and off the grid is signal stabilization. The wind comes and goes making the turbine spin at different speeds at almost every instant. In order to be useful for the grid, or the interconnected network of electricity produced, it must be consistent and stable. Most cases batteries are added in between the turbine and the grid to stabilize the electricity produced. They are not the most efficient at doing so but they are the most cost effective.
This signal stabilization is done by charging and discharging the battery at the same time. Although it is not very advantageous, lead acid batteries can be charged and discharged at the same time. Wind moves the turbine and sporadically charges the battery. The load, or whatever is using the electricity produced is hooked up to the battery and discharging it. The battery charge will go up if the turbine produces more energy than used and vice versa. If the constant charge and discharge is done in a certain threshold in the batteries charge percentage it can last longer than if it is done with a higher or lower charge. Due to the near impossibility of predicting the wind, it is very difficult to build a system that maximizes the life of the batteries but still performing the stabilization. Complicated systems are being built to replace the batteries. But for now, the battery acts as the bridge between the wind energy and the grid. By implementing a battery in the system the fluctuations decrease by 2.5%. (Seoud, Jatskevich 2008)
This fluctuation stabilization can lead to consistent replacement of the batteries but Like the photovoltaic system the lead acid battery is the most cost effective but not the ideal battery for use. Batteries are configured in different series and parallel configurations within the system based upon a quality of the batteries known as internal resistance. Different size batteries have different internal resistances. These batteries are configured very specifically to make an ideal internal resistance for the system.
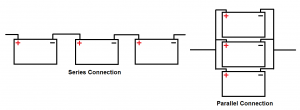
The two ways the batteries can be implemented into the system is in “series” and in “parallel”. A series connection puts the batteries in a train like structure. They are connected to each other by one wire this creates only one path for the electricity to flow. The parallel connection hooks them up side by side using multiple wires. This allows for the electricity to flow in more than one path. A series connection within the batteries makes the internal resistances add together creating a higher internal resistance of the system, while a parallel connection divides the internal resistances into a smaller number. An easy analogy would be to think of driving on a road: The series connection is a one lane road that is easily blocked. The parallel connection has multiple lanes and allows for the flow to be much easier even if one lane is blocked.
Figure 2
Two different ways batteries can be connected together.
Like the photovoltaic systems the wind systems are also limited by the batteries used inside them. The lead acid batteries used in storage and stabilization inside the system have the shortest lifespan and need replacing more than any other component. The system is sporadic and unpredictable making it harder for the system to be modified to maximize the battery life. If another way of stabilizing the electrical system could to replace the batteries, the system would be much more cost effective.
Conclusion
Although battery technology has vastly improved in recent years, the push was mainly for more power in small spaces. This is specifically advantageous for low small handheld electronics, but larger power applications are still using old lead acid technology. Unlike the newer batteries such as lithium ion, lead acid batteries still used liquids inside to create electricity. These chemicals are very dangerous and damage the environment if not disposed of correctly. They are much larger than new technology and exponentially heavier due to the use of the lead. They cannot be completely discharged like new batteries can and do not have as long of a lifespan. Only because of their easy production methods are they still in use today. Scaling up a newer battery technology to the power of a lead acid is not worth the price. Alternative batteries need must be produced to continue the growing renewable energy industry and cut its costs.
Solar and wind power require batteries because of the inconsistency of power being produced. This power inconsistency can be seen in most other renewable energy applications as well. Wave power generates power using the ocean waves needs these batteries to store the energy is produces every few seconds. Tidal power that creates electricity much like the wind power needs batteries to stabilize as well as store energy. These renewable sources of power will always be in need of battery storage elements. In order to implement these more better batteries must be used to lessen the cost and maintenance.
With the lithium ion battery needing to be specifically designed for such a large application other battery technologies are being tested in renewable energy application. The ultracapacitor is being considered as an option. The ultracapacitor has a much longer lifespan then the lead acid battery. It does not contain any liquids and does not rely on any chemical reactions. It can be completely discharged without problem and can be stored in any state. The charges can flow in and out much faster so it can be charged at a much faster rate (Glavin, Hurley 2006). This results in the system being more efficient and the batteries lasting much longer than before. Although these batteries have the ideal characteristics for renewable applications, they need must be mass produced and cost effective before they will be widely implemented.
Bibliography
- Aboul-Seoud, T., & Jatskevisch, J.. (2008) Improving Power quality in remote wind energy systems using battery storage. Electrical and Computer Engineering, 2008. CCECE 2008. Canadian Conference 4-7 may 2008. DOI: 10.1109/CCECE.2008.4564842
- Al-Sheikh, Hiba, & Moubayed, Nazih. (2012). Health Status and Diagnosis of Batteries in Renewable Energy Systems: An Overview. 2012 International Conference and Exposition on Electrical and Power Engineering (EPE 2012), 25-27 October, Iasi, Romania. DOI: 10.1109/ICEPE.2012.6463814
- Glavin, M., & Hurley W.G.. (2006) Battery Management Systems for Solar Energy Applications Universities Power Engineering Conference, 2006. UPEC ’06. Proceedings of the 41st International (Volume:1 ),. DOI: 10.1109/UPEC.2006.367719
- Parsekar, Sachin & Chatterjee Kishore. (2014) A Novel Strategy for Battery Placement in Standalone Solar Photovoltaic Converter System Indian Institute of Technology. DOI: 10.1109/PVSC.2014.6925498
Suggested Reading
- Optimisation of a photovoltaic battery ultracapacitor hybrid energy storage system. DOI: 10.1016/j.solener.2012.07.005
- Lithium-Ion Capacitor Energy Storage Integrated With Variable Speed Wind Turbines for Power Smoothing DOI: 10.1109/JESTPE.2013.2284356