Developing a Long Term Bioelectric Interface for Measuring Multiple Human Neurons
by Aiden Lewis
Mentor: Brian Timko, Biomedical Engineering; Funding Source: Nathan Gantcher Student Summer Scholars Fund
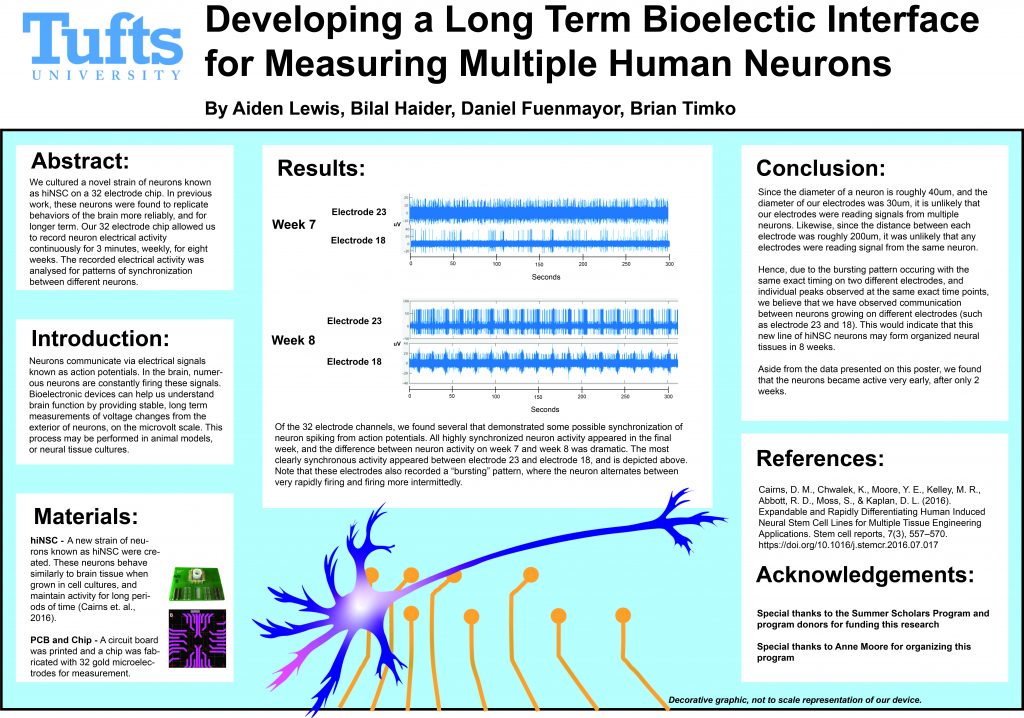
What goes into measuring the voltage of a neuron?
The body of a neuron is about 40um in diameter. This is less than the width of an average human hair. Accordingly, a typical electrical signal from a single neuron is approximately 40mV, or 0.44% of the electrical potential difference in a 9V battery. Additionally, this refers to the electrical signal if measured from inside of the cell. When we attempt to measure the electrical signal from outside of the cell, in order to avoid damaging it, the signal drops down in orders of magnitude, to about 40 microvolts, which is one one-millionth of a volt. Aside from being tiny voltages, each action potential is extremely fast, and occurs over approximately 5 milliseconds.
In the Timko lab at Tufts, we fabricate specialized electronic devices that are capable of measuring these tiny biological signals. Our goals are to design devices that are not hazardous to the cells, capable of high quality electrical readings (high signal to background ratio), and have a high capacity for doing multiple measurements at once. The ideal device would thus allow us to measure the activity of many neurons at once, obtaining high quality continuous data for long periods of time.
The 32-electrode device we used for this long term measurement (8 weeks), of a new human neural stem cell line, was fabricated with methods typical of devices previously used in the Timko lab. This neuron line was known to develop quickly, and reliably behave like neurons in an active brain (Cairns et. al., 2016). The fabrication process for our chip involved sputtering a thin film of chromium or titanium and gold onto a perfectly flat silicon wafer. This is a process where plasma is used to strike a source of metal, which deposits thin films of the metal in precise locations to create an electrode pattern on our flat silicon base. Next, the silicon chip is attached with conductive silver epoxy to a printed circuit board that is wired to our data collecting devices. The electrical signal from the chip is sent to an amplifier, which makes the electrical signals larger and easier to read, then, the electrical signals are sent to an analog to digital converter, which essentially records data points of voltage at 50,000 samplings per second. This means we can distinguish the strength of electrical signals from one electrode every 0.02 milliseconds. This effectively means we get 250 time points for recording the average 5 ms neuron signal, so we can see them in good detail.
Finally, a circular plastic well was attached to the surface of the chip, and neurons were grown in the chip, using media that mimics the nutrients they prefer inside the body. The neurons were grown in an incubator at 37 degrees C, and their electrical signals were measured once a week for 5 minutes at a time.
My work over the summer concerned remote analysis of these electrical signals. When looking at the electrical signals, I focused on identifying “spikes”, which are sharp jumps in voltage that correspond to electrical signals inside the neurons. Over the course of the experiment, we recorded thousands of “spikes” from the neurons. At their most active, our neurons were spiking over 1000 times per minute. In my analysis, I compared the timing of these spikes from electrode to electrode, looking for possible coordination between neurons located on different parts of the chip. Thankfully, in the last week of the experiment, many electrodes showed similar timing, which implied possible communication between neurons.
The field of brain-machine interfaces is rapidly developing, especially with some high-profile investment from figures like Elon Musk, and Softbank. The goal of such devices is to create a seamless, non-invasive device that can measure high quality recordings from numerous neurons at once, and even signal back to those neurons. In the future, we hope to develop more sophisticated and flexible electrode arrays that can be wrapped around 3D gel cultures of neurons (Min et. al., 2014). This would allow us to better model brain activity, which could lead to new discoveries in neuron physiology, including better understanding of the mechanisms behind some neural diseases such as parkinson’s (Ellens et. al., 2013). What kind of brain-machine interfaces excite you? What ethical fears might you have about the potential of this research? What good do you want to see come out of electrical brain-models?
References:
Cairns, D. M., Chwalek, K., Moore, Y. E., Kelley, M. R., Abbott, R. D., Moss, S., & Kaplan, D. L. (2016). Expandable and Rapidly Differentiating Human Induced Neural Stem Cell Lines for Multiple Tissue Engineering Applications. Stem cell reports, 7(3), 557–570. https://doi.org/10.1016/j.stemcr.2016.07.017
Ellens, D. J., & Leventhal, D. K. (2013). Review: electrophysiology of basal ganglia and cortex in models of Parkinson disease. Journal of Parkinson’s disease, 3(3), 241–254. https://doi.org/10.3233/JPD-130204
Min D. Tang-Schomer, James D. White, Lee W. Tien, L. Ian Schmitt, Thomas M. Valentin, Daniel J. Graziano, Amy M. Hopkins, Fiorenzo G. Omenetto, Philip G. Haydon, David L. Kaplan (2014) Three-dimensional brain-like tissue. Proceedings of the National Academy of Sciences, 111 (38) 13811-13816; DOI: 10.1073/pnas.1324214111
Hi Aiden, the idea that you are working to design a device that can measure electrical activity in the brain is fascinating! I think this project is really cool, and I am excited to hear more about this.
Your findings were just as interesting to learn about the second time around. Great work!
This research is insane!! I never knew these things were possible. Amazing research and an amazing poster! The results and conclusion sections were concise and clear.