Our Research
Interaction of bacterial pathogens with host cells
Our general interest is in the interaction of bacterial pathogens with mammalian cells, interactions that promote colonization and trigger immune responses that can enhance tissue damage and/or result in clearance of the pathogen.
Enterohemorrhagic Escherichia coli
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 utilizes a specialized type III secretion system that injects bacterial “effectors” into mammalian cells to colonize the colonic mucosa, and produces the potent phage-encoded Shiga toxin, which damages the intestinal epithelium and, in some patients, results in systemic vascular damage and renal failure. Antibiotic treatment can induce production of the phage-encoded toxin and is contraindicated during EHEC infection, so we lack specific therapies to prevent or treat EHEC infection and disease. To investigate mechanisms of disease and generate new methods to fight EHEC disease, we developed an EHEC infection model utilizing the natural murine pathogen Citrobacter rodentium, which colonizes the colonic epithelium similarly to EHEC, (Fig. 1; Mallick et al., 2012; Flowers et al.,2021). In collaboration with Drs. Cheleste Thorpe (Tufts Medical Center), Priya Kailasan Vanaja (UConn Health School of Medicine), and Olga Kovbasnjuk (University of New Mexico), we are also utilizing the interaction of EHEC and Shiga toxin with colonic epithelium utilizing stem cell-derived colonoid models. Our goal is to generate means to block colonization or disease by targeting bacterial or host factors related to the function of the type III secretion system or the production or action of Shiga toxin. These studies may provide approaches to address other intestinal infections or disorders.

Figure 1. C. rodentium colonizes the murine colonic mucosa. GFP-producing bacteria (courtesy of I. Rosenshine) are localized on the epithelium, for which the nuclei are stained blue with DAPI and filamentous actin is stained red with phalloidin.
Borrelia burgdorferi, the Lyme disease spirochete
The Lyme disease spirochete Borrelia burgdorferi sensu lato, carried by the Ixodes tick, is the most common vector-borne pathogen in the U.S. In the days to weeks following the establishment of a local skin infection at the site of the tick bite, this bacterium can spread to the heart, joint and other tissues. Lyme disease strains differentially colonize different tissues, leading to diverse manifestations of disease. We utilize a variety of methods, including genome-wide screens (a collaboration with Drs. Wolf Zückert [University of Kansas] and Brandon Garcia [East Carolina University]) and targeted mutagenesis, to identify and investigate spirochetal factors that promote bloodstream survival and tissue colonization with a particular focus on factors that influence dissemination kinetics and the tissue tropism (in collaboration with Drs. Jenifer Coburn [Medical College of Wisconsin] and George Chaconas [University of Calgary]). We have found that the spirochete encodes surface proteins of variable sequence that modulate activation of the host complement system and influence the sites of colonization (Lin et al., PLoS Pathogen ‘20).

Figure 2. B. burgdorferi produces CRASPs, i.e. surface proteins that bind host complement regulatory proteins. Wildtype B. burgdorferi, stained with Hoechst (blue), bound to the host complement regulator Factor H (FITC-labeled, green), which localizes to the bacterial poles.
Streptococcus pneumoniae
Invasive pneumococcal disease caused by Streptococcus pneumoniae is responsible for over a million deaths per year worldwide. Lung infection, one of the most common forms of invasive pneumococcal disease, is associated with a robust influx of polymorphonuclear cells (PMNs) into alveolar spaces. This acute inflammatory response causes significant tissue damage and contributes to pneumococcal disease. By establishing a variety of pulmonary epithelium infection models (Fig. 3), we have identified pathways by which S. pneumoniae triggers PMN influx into the airways (Fig. 4) and have shown pneumolysin, a cholesterol-dependent pore-forming toxin, induces PMN migration across pulmonary epithelium ([Adams et al, 2020], a collaboration with Dr. Rod Tweten [Oklahoma University Health Science Center], Hongmei Mou [Mass General Hospital], and Michael Mansour [Mass General Hospital]). We also utilize murine infection models to validate and expand upon our in vitro models.

Figure 3. Rapid peri-bronchial accumulation of infiltrated neutrophils during pulmonary S. pneumoniae infection. PFA- fixed lung thick section, neutrophils (Ly6G, blue); bronchial epithelium (E-cadherin, Yellow); Actin (Phalloidin, red); Nucleus (DAPI, white). (A collaboration with Dr. Shumin Tan, Tufts University School of Medicine.)
Importantly, neutrophil transmigration severely damages epithelial integrity and promotes the spread of the pathogen into the bloodstream. Thus, mice diminished in the recruitment of PMNs to the airway space are paradoxically protected lethal systemic disease by S. pneumoniae. Finally, this pathogen causes particularly severe disease in elderly individuals and in those co-infected with influenza virus. Inflammatory responses are altered upon aging or viral co-infection, and in collaboration with Drs. Joan Mecsas (Tufts University School of Medicine), Simin Meydani (Friedman School of Nutrition), Megumi Inomata (Asahi University School of Dentistry), Jennifer Philips (Washington University School of Medicine) and Elsa Bou Ghanem (University of Buffalo), we are utilizing both tissue and murine models to investigate these susceptibilities with the goal of mitigating systemic disease (Bou Ghanem et al., 2017; Inomata et al., 2020).
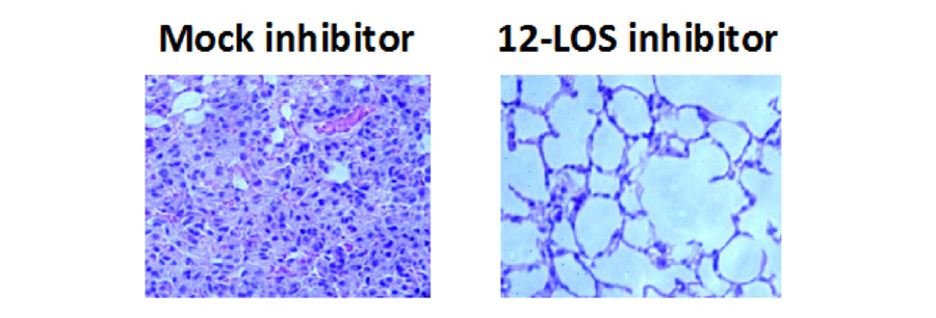
Figure 4. Mice that had been mock-treated or treated with a 12-LOS inhibitor, which blocks the production of the neutrophil chemoattractant hepoxilin A3, were infected intratracheally with S. pneumoniae, and lungs were H&E stained after 2 days of infection [Bhowmick et al, 2013].
