Separations Activity
Introduction & Motivation
When chemical engineers use reactions in their work, often times they produce undesirable molecules on the side. Many industrial processes involve separating mixtures to purify a certain target, meaning that engineers have to understand the properties of the possible components in order to succeed.
There are many properties to a substance! Some things are water-soluble (dissolve well in water,) while others might be soluble in alcohols or other organics. Sometimes a component will have a low boiling point, so the mixture can be heated and the vapors collected. Sometimes the mixture can be put in a machine called a centrifuge, which spins samples like a really fast merry-go-round to push solids to the bottom of the test tube. The challenge for chemical engineers is then to decide what properties to take advantage of during separation: boiling point, density, particle size, chemical properties, to name a few. This activity will allow students to categorize the components of a mixture based on their properties and take advantage of them by experimenting with different separation and filtration techniques.
Materials
- Sea salt
- Sand
- Iron filings
- Beads
- Coffee filters
- Small magnets
- Plastic cups
- Plastic plates
- Plastic bags
- Rubber bands
- Tray to do project on in case of spills
- Beakers/crucibles
- Hot plate
- Teaspoon and tablespoon measures
- Mass balance and weigh paper (optional)
Procedure
Before the experiment, prepare bags of mixed ingredients. Measure out the masses of iron, sand, and salt you are adding to the bags and write the masses of each on the bag. (Start with 1 tbsp sand, 1 tsp salt, 1/8 tsp iron, 1 tbs baking soda, 20-30 beads). Make sure to have extra bags so that if the students want to try a different order or method of separation, they can get a new bag to try again.
Tell students what the mixture in the bag is. First, ask them about the different properties of the materials in the bag and how they could use these properties to separate the materials, then split them into groups and have them perform the separation. The ideal separation method is described below, though allow students to develop a separation procedure on their own.
Challenge: Add some baking soda to the mixture and include vinegar as a tool for separation. This is a great place to talk about how important chemical separations can be in purifying a subject as well!
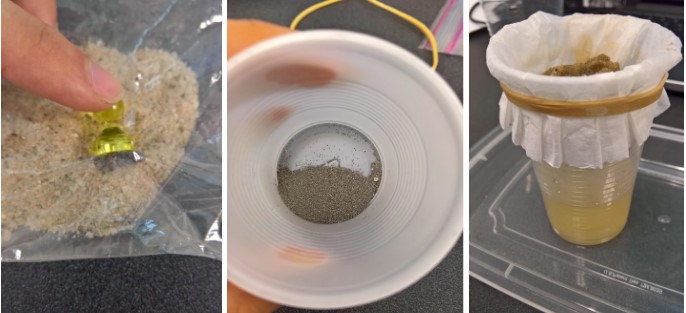
- First, the magnet will be moved around on the top of a plastic bag to accumulate the iron filings. The clump of filings can then be removed from the mixture.
**Tip! Be sure not to touch the iron filings directly to the magnet, as they are very difficult to recover afterward!** - The remaining mixture can be mixed with water. The beads will float and can be effectively skimmed off.
- The remaining solution can then be put through a coffee filter which is rubber banded to a plastic cup. The water will dissolve the salt and the sand will get stuck in the filter while the salt water flows through. The sand can be dumped into a beaker or crucible to be dried on a hot plate.
- The salt water can then be boiled in a beaker or crucible on a hot plate, leaving behind the salt crystals.
- (Optional): Have students mass their separated ingredients to see how close to the original mass they recovered!
Discussion
- Ask students how they separated the three materials. This should involve discussion about the properties of the materials. Why does the magnet work on the iron but not the sand?
- Was the separation perfect? Did you recover exactly the same mass as was recorded on the bag? What could have interfered with this?
- Do the order of the processes matter? Can you separate the sand before everything else? The salt?
- What problems came up along the way? How can these be remedied? (Sand getting through the filter could be remedied by using finer filters, using multiple at once, or going through multiple rounds of filtering.)
- Was the filter fouled? (i.e. could it be used again effectively?)