Experiments

Silk Nanoparticles
Silk Processing
Cocoons were cut and weighed with sodium carbonate and added to 2L of boiling distilled waters to degum and leave only silk fibrin. Washed degummed silk was then dried in a fume hood. Silk is then added to a LiBr solution to remove beta sheets. Dissolved silk is added to dialysis tubing in distilled water and put for 3 days. Silk solution is final centrifuged and stored for use.
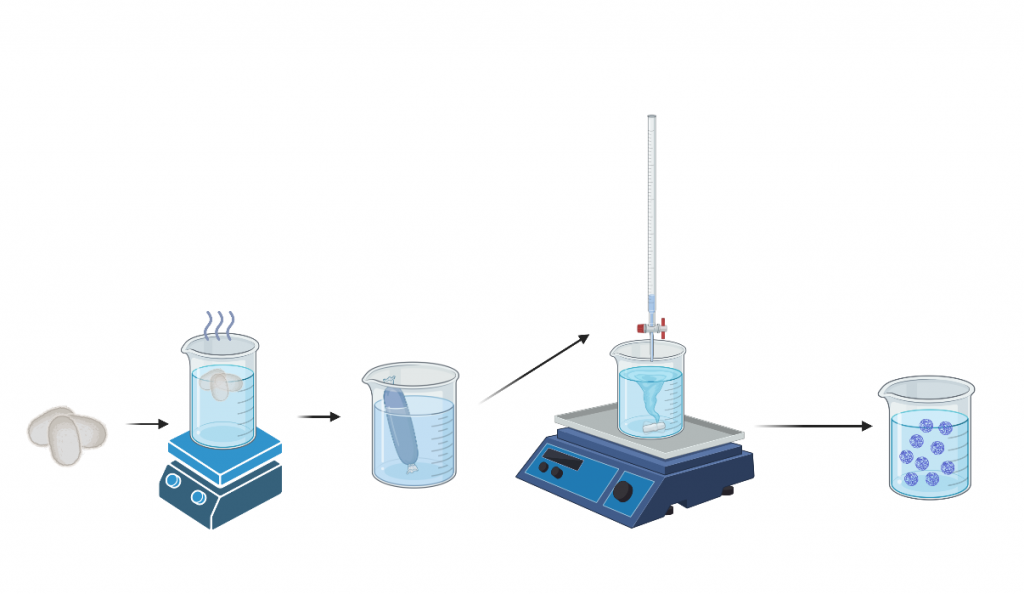
Nanoparticle Formulation
Acetone was added to acetone in a small glass jar. Next we added a stir bar and secured the jar onto the center of the stir plate with polymer clay, set to 500 rpm. The vortex created was centered and no sound was coming from the stir bar. Measure out 4 mL silk & pour it into a specialized glass dropper. Using titration technique to slowly drop silk solution into acetone. The solution should fall into the side of the vortex to create the desired nanoparticle size of choice. The jar should spin for 3 days, with DI water added on day 2. On day three the nanoparticle solution was sonicated at 30% amplitude for 30 seconds. Nanoparticle size was then confirmed using Dynamic Light Scattering (DLS).
EDC/NHS Antibody Conjugation
EDC/NHS Conjugation
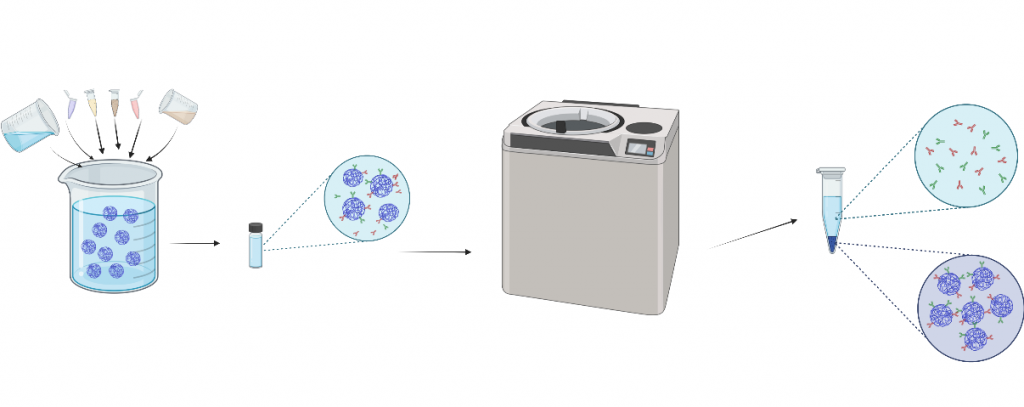
EDC and NHS powders were weighed out into small individual jars, respectively. Based on calculations, MES buffer (pH 6) was added to help dissolve the EDC and NHS separately. We then performed calculations to determine the amount of ultrapure water that will be added to the dissolving process. In a new jar with a stir bar, add silk NPs, MES buffer, EDC +NHS, and the antibody. Set the jar on a stir plate at 200 rpm for 18 hours overnight. On day 2, two tubes with EDC/NHS nanosolution were filled equally and weighed, using DI water to balance.
Ultracentrifugation
EDC/NHS solutions were ultracentrifuged three times to wash unbound antibodies. The supernatant was taken out with a needle and resuspended with DI water. Supernatant pellets were soaked in 1 mL of DI water and stored in the freezer.
Secondary Antibody Tagging
Secondary Antibody Tagging
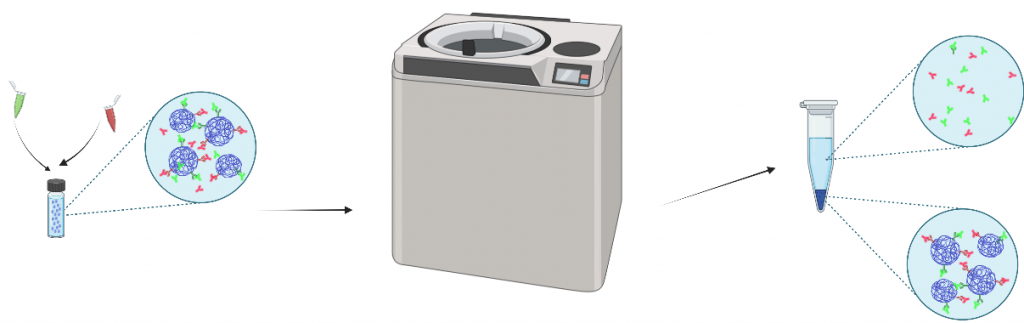
Secondary antibody tagging was used to determine successful EDC/NHS antibody conjugation and orientation. Nanoparticles are resuspended in a sealing tube and sonicated. A total of 5 tubes will be generated using nanoparticles, blocking buffer, and secondary antibodies. These 5 tubes will be shaken on a stir plate covered in tin foil for 2 hours before following the Ultracentrifugation protocol again to wash out unbound secondary antibodies.
Fluorescent imaging
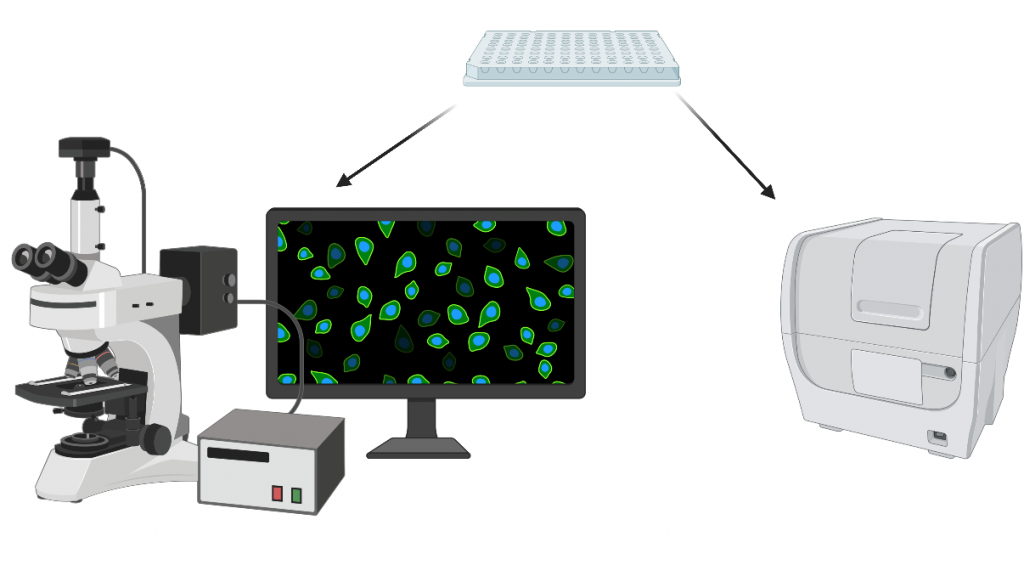
In order to validate the antibody conjugation, we used fluorescence microscopy and a 96-well plate reader. For each new secondary antibody, we created a standard curve. Controls were used to control for silk autofluorescence and non-specific secondary antibody coating.