Results
Proof of Concept Single IL-4 Conjugation
- IL-4 conjugated silk nanoparticles had significantly higher antibody concentrations than controls
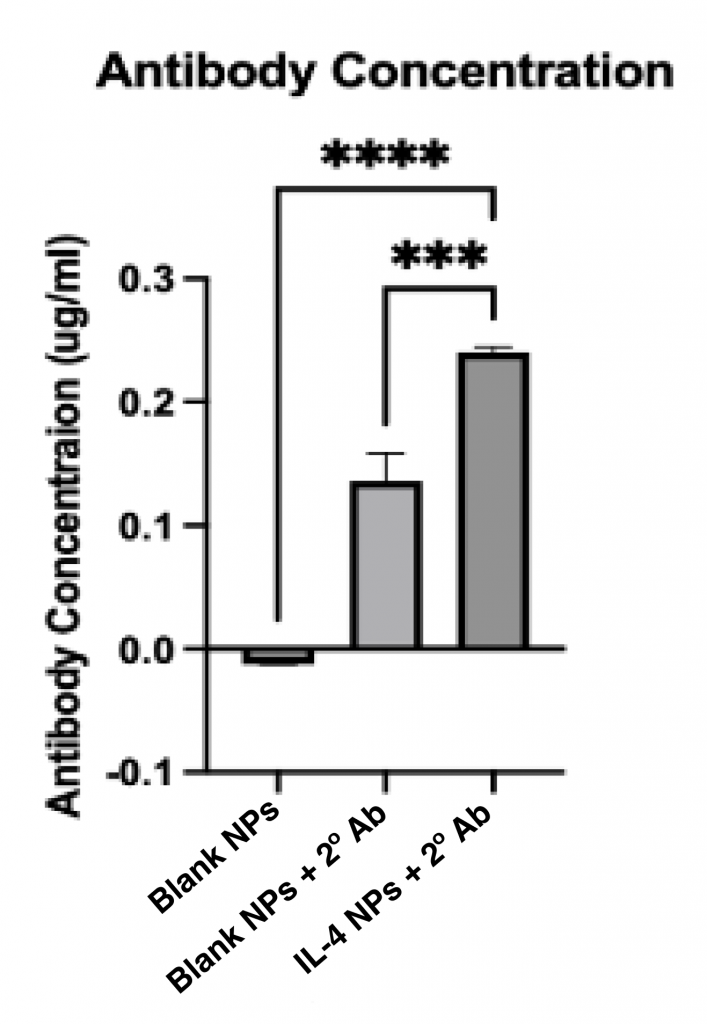
Conclusion: Successful IL-4 conjugation to silk nanoparticles
Single and Dual PSTAT3 and anti-rVEGF Silk Nanoparticles
- Successful EDC/NHS single conjugation of PSTAT3 and anti-rVEGF with significantly higher antibody concentrations than controls (Figure 4B and 4D)
- EDC/NHS dual conjugation of PSTAT3 and anti-rVEGF had significantly higher antibody concentrations than controls (Figure 4A and 4C)
- Keyence Microscope images showed high levels of colocalization of anti-rVEGF and PSTAT3 (Figure 3 and Table 1)
Conclusion: High levels of colocalization predict dual antibody conjugation. Successful single conjugation of PSTAT3 and anti-rVEGF to silk nanoparticles
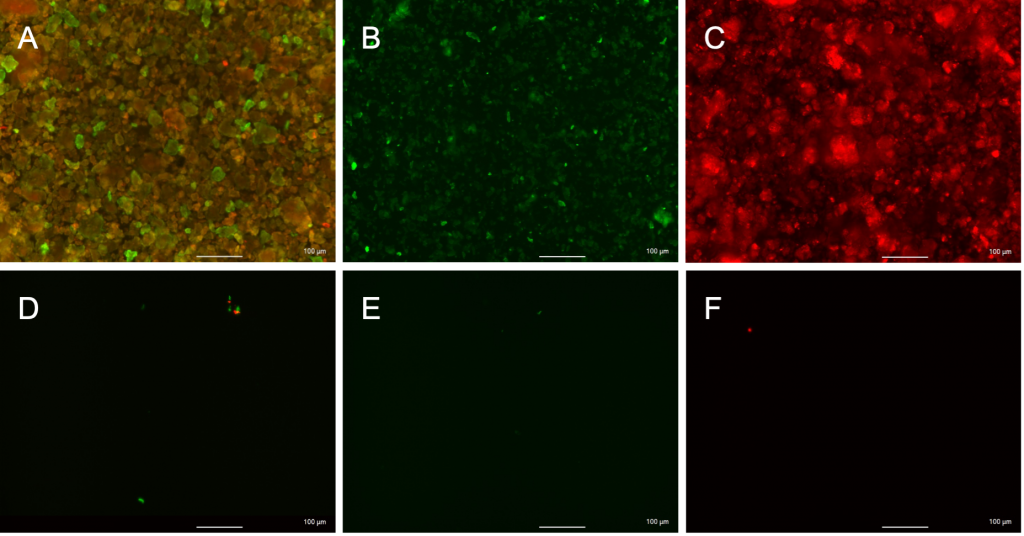
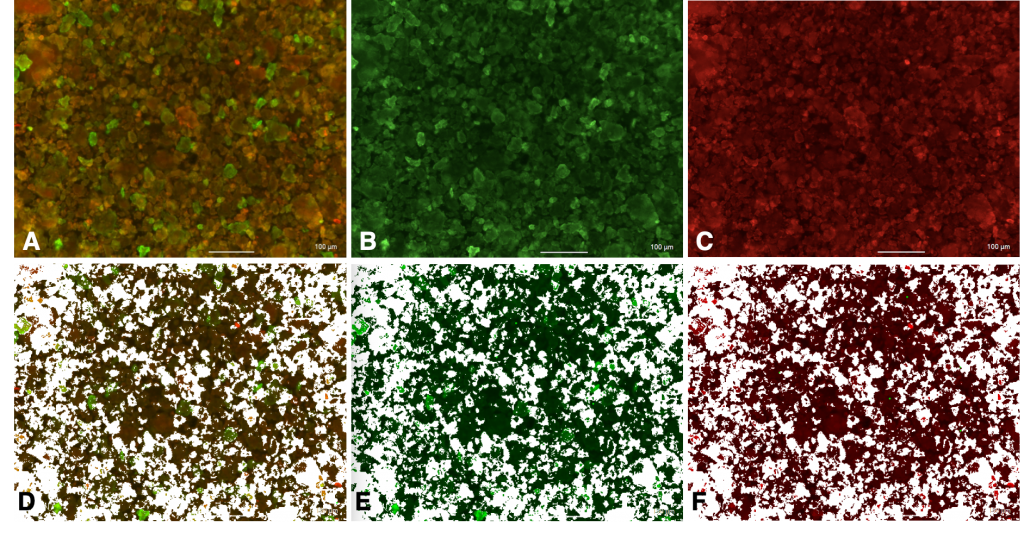
| Area of PSTAT3 | 39.326 |
| Area of anti-rVEGF | 38.826 |
| Area of overlap | 31.842 |
| overlap/PSTAT3 | 80.969% |
| overlap/anti-rVEGF | 82.012% |
Table 1. ImageJ Analysis of images in Figure 3 calculated relative areas of overlap as well as green and red fluorescent signals for PSTAT3 and anti-rVEGF respectively. 80.969% of PSTAT3 was colocalized with anti-rVEGF, and 82.012% of anti-rVEGF was colocalized with PSTAT3.

How did you determine successful antibody binding?
We determined that primary antibodies successfully conjugated in the correct orientation when secondary antibodies were able to bind to the antigen site on the primary antibody and fluoresce. The fluorescence was then checked against blank nanoparticles and blank nanoparticles incubated with secondary antibodies to confirm that any fluorescence was only due to proper secondary binding and not influenced by silk autofluorescence or non-specific secondary coating respectively. Antibodies that had improperly conjugated would not have their binding site available for secondary binding thus they would not fluoresce.
What are some limitations to this work?

- Nanoparticles observed during imaging were still aggregated despite several rounds of sonification, hindering the characterization of singular nanoparticles.
- Clinical relevance was unable to be established during this round of experimentation as timing did not permit for cell uptake studies.
- Future replicate studies are needed for stronger data
However, both of these points can be ameliorated with future work!
Read a more thorough explanation of the results in our Technical Report: