Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008 Mar;11(3):354-9. doi: 10.1038/nn2046. Epub 2008 Jan 27. PMID: 18223647; PMCID: PMC2655319.
Introduction: This paper explores the connection between GABA activity and insomnia. As discussed before, the causes of insomnia are not known for certain, but many bodies of research have suggested that there is a link between GABA and human sleep regulation. This paper supports that theory by demonstrating a link between the GABA-A receptor and sleep latency in Drosophila Melanogaster.
Background: Drosophila Melanogaster were discovered to have a mutated GABA receptor, denoted RDL. This mutation causes the receptor to have a single long channel constantly open, promoting the ion gradient of chloride ions to be constantly used, which decreased sleep latency (the time it takes to fall asleep). Fruit flies have GABA receptors very similar to mammalian receptors in terms of structure and function, making them great subjects for studies into the link between GABAergic activity and sleep. The authors decided to focus on this mutated receptor by monitoring the sleep patterns of wild-type and mutated drosophila. The authors hoped that any possible trends discovered in the study could be reasonably applied to mammalian sleep cycles.
Methods: The authors cultured wild-type and mutated flies at a well-regulated 12-hour light-dark cycle in an agar medium. After having been cultured for 5-7 days, flies were transferred to a machine-regulated 24-hour light-dark cycle and their locomotor activity was measured. Locomotor activity was analyzed every minute. Sleep was characterized as five straight minutes of inactivity. Sleep latency was denoted as the time between “lights off” and the first sleep episode.
Results:
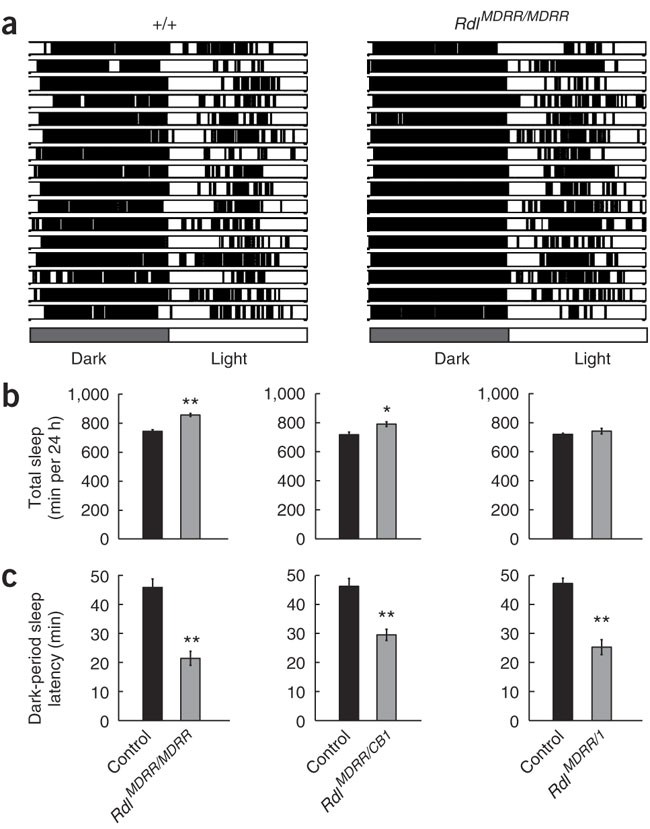
The authors collected data from the sleep patterns of two populations of flies, displayed in Figure 1. At the top, the bar graphs represent the sleep durations for each fly in either population, with black bars representing sleep and white bars representing wake periods. Clearly, the mutation proved to decrease the sleep latency and also led to uninterrupted sleep in the mutated flies. This can be seen as the periods of sleep (denoted by the black lines) during dark conditions are much more uniform in the mutated flies. The data confirm that the mutated GABA receptor has an effect on sleep regulation.
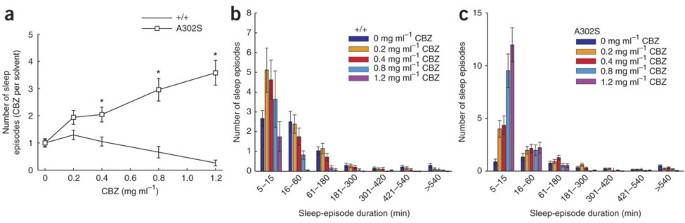
Beyond this, the authors utilized a sleep-depriving drug called carbamazepine, or CBZ. CBZ, a three-membered ring, is similar in structure to the class of drugs known as benzodiazepines. CBZ is an agonist to the GABA receptor and is often used to treat epilepsy. While the expectation would be that CBZ would help decrease sleep latency (similar to how benzodiazepines are used to treat insomnia), CBZ actually has been shown to increase sleep latency and inhibit GABAergic neuron signals through a currently unknown mechanism. In fact, many studies in humans have shown that those receiving CBZ-therapy often experience sleep problems. The authors administered this drug to both the wild-type and mutated flies to see if there were similar effects. As seen in Figure 3, the mutated flies with their mutated RDL receptor were able to establish homeostasis in response to CBZ concentration, which increased sleep latency and decreased sleep duration in wild-type flies. Note how in all three graphs, the mutated flies consistently had more sleep episodes in high concentrations in CBZ compared to wild-type flies.
Conclusion: From this data, the authors were able to conclude a relation between GABA and GABA-A receptors and sleep regulation. The RDL mutation largely removed the GABA regulation from the GABA-A receptor, as the mutation caused an “always on” response that greatly improved sleep latency. When both wild-type and mutant flied were treated with CBZ, the mutant flies were able to counteract its effects. This suggests that CBZ was limiting GABA-A kinetics in the wild-type receptor, which means that the GABAergic signals that are normally associated with this receptor are essential to sleep regulation. Additionally, the similarity of responses to CBZ in the flies in this study and humans in other studies illustrates the potential of using fruit fly models to study GABAergic signals in humans. Thus, the authors confidently concluded a relation between GABAergic signals and sleep regulation in drosophila melanogaster. As human sleep and fruit fly sleep have similar mechanisms, similar mutations to GABA receptors (which have been associated with insomnia) can be used to confirm this relation in humans. Furthermore, targeting different areas of the GABA receptor (analogous to the mechanism of CBZ) would likely provide different therapeutic pathways for sleep disorders in the future.
VERY VERY well done. I love the connection back to GABAa receptor pathway and further support the relationship between the receptor and insomnia/sleep recognition. I also love on the elboration of carbamazepine and its possible effects. However, I was also surprise to find that carbamazepine increase sleep latency who has similar structure to benzodiazepines. Very interesting article and very well done.
Have you read any papers investigating the neural pathways that affect the mis regulation of the NT’s in the brain and result in depression?
I ask this because i would be curious if light sensitivity results in GABA abnormalities.
Good analysis of this figure and selection of this as drosophila are a powerful model organism for studying various metabolic pathways and have strikingly high conservation to human genomes.
This page does a great job selecting figures and explaining how GABA receptors regulate sleep/wakefulness. What other effects does the mutated GABA receptor cause? Does it affect other pathways or phenotypes? I also wonder if GABA production is affected by light exposure like melatonin is and if the flies could adapt to this mutation long term.
These are great questions (and I think Robert had a similar question above). I didn’t read too much else about this mutated GABA receptor but it’s definitely an interesting area of research. I also don’t know much about the link between GABA and light exposure, but that would be a cool connection between our two projects!
This page was so good it almost made me cry. Well done lad.
yessir thanks my guy
Nicely done here. One suggestion I have is to go more into depth about the actual data obtained from the experiments and how those data relate to the conclusions made at the end. Also, there are a few typos throughout the text, so just read closely before the deadline to remove those. Otherwise, I thought this was a very clear analysis, and your conclusion made it easy to understand what to glean from the experiment.
Great idea, I added some more connections to the data in the figures (without adding so much that it would detract from the overall paper) and I fixed the wording.