Research
Investigate the mechanisms of host preferences to maintain and spread Lyme disease bacteria
The efficiency to be maintained in reservoir animals vary among different species or strains of Lyme disease bacteria, but the mechanisms that lead to such differences are unknown. In this study, we introduced different bacteria species/strains into reservoir hosts (white footed mice and American robins) or the avian and mammalian model animals (house mice and quail). We have shown that the host-to-host different ability to carry Lyme disease bacteria is determined by the variation of host immune responses (specifically, complement, antibody, and inflammatory responses) and/or polymorphisms of pathogen proteins that confer cell attachment and immune evasions. We also further defined several bacterial proteins as the determinants to contribute to transmission and long-term infection and identify the convergent evolution as the mechanism arisen through such host preferences to maintain and spread Lyme disease bacteria. Defining such mechanisms would impact the development of preventive strategies, allowing us by tweaking those bacterial determinants in the vaccines or prophylaxis to trigger similar immune responses in the incompetent hosts that would not allow bacterial survival. In this project, we are collaborating with Drs. Klemen Strle in Tufts Medical School, Maria Diuk-Wasser in Columbia University and Sergios Kolokotronis in SUNY downstate Medical Center for the infection of white footed mice and phylogenic analysis.
Develop the effective Lyme disease preventions and identify their protective mechanisms
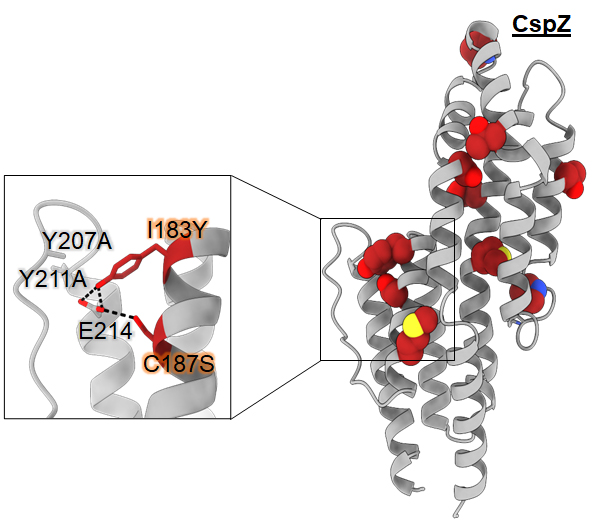
Using the mechanisms elucidated above, we revised the sequences of a previously shown ineffective vaccine antigen, CspZ, to improve the protective antibody responses. We have shown that our patented invention for this revised new vaccine (CspZ-YA) can effectively prevent mice from the infection caused by multiple strains/species of Lyme disease bacteria. Additionally, we also examined the potential of two agents (FH-Fc and non-anticoagulant heparin) invented by our collaborators as pre-exposure prophylaxis and showed eradications of Lyme disease bacteria by those agents in vitro and/or in vivo. We further identified the modulation of antibody responses, complement-mediated killing, and adhesion blocking by these agents as their protective mechanisms against Lyme disease bacteria (see the above Figure, left panel). Examining the efficacy and identifying the protective mechanisms would impact the generation of prevention strategies to eventually reduce the burdens of human Lyme disease. In this project, we are collaborating with Drs. Maria-Elena Bottazzi in Baylor College of Medicine, Robert Linhardt in Rensselaer Polytechnic Institute, and Planet Biotech, Inc. to test the efficacy and define the protective mechanisms of those vaccines and prophylaxis.
Define the role of tickborne co-infections in enhancing the Lyme disease severity.
Antibiotic therapy is often effective for Lyme disease and tickborne coinfections, but some patients experience long-lasting complications for unknown causes. The ticks that carry Lyme disease bacteria can also transmit other pathogens, including species of Ehrlichia, Babesia, Anaplasma. Patients with Lyme disease and other tickborne diseases often exhibit more severe Lyme disease-associated manifestations, but the mechanism that drives such enhanced severity is unknown. Using Lyme disease bacteria and Ehrlichia (and Babesia) as a model of tickborne coinfections, we have shown the role of less robust early innate myeloid cells to enhance the burdens of Lyme disease bacteria during coinfections with Ehrlichia, which is linked to the increasing severity and pathogen dissemination in joints at late stages of coinfections (see the above Figure, right panel). These results would impact the development of new therapeutics for tickborne coinfection. The key players of immune responses identified in this work to promote bacterial dissemination and Lyme disease-associated manifestations during co-infection could be modulated as a new therapeutic strategy. In this project, we are applying our mouse model of tickborne coinfection to examine such a concept in my laboratory, with the collaboration from Dr. Kate MacNamara in Albany Medical College to provide the characterization of the immunological mechanisms.