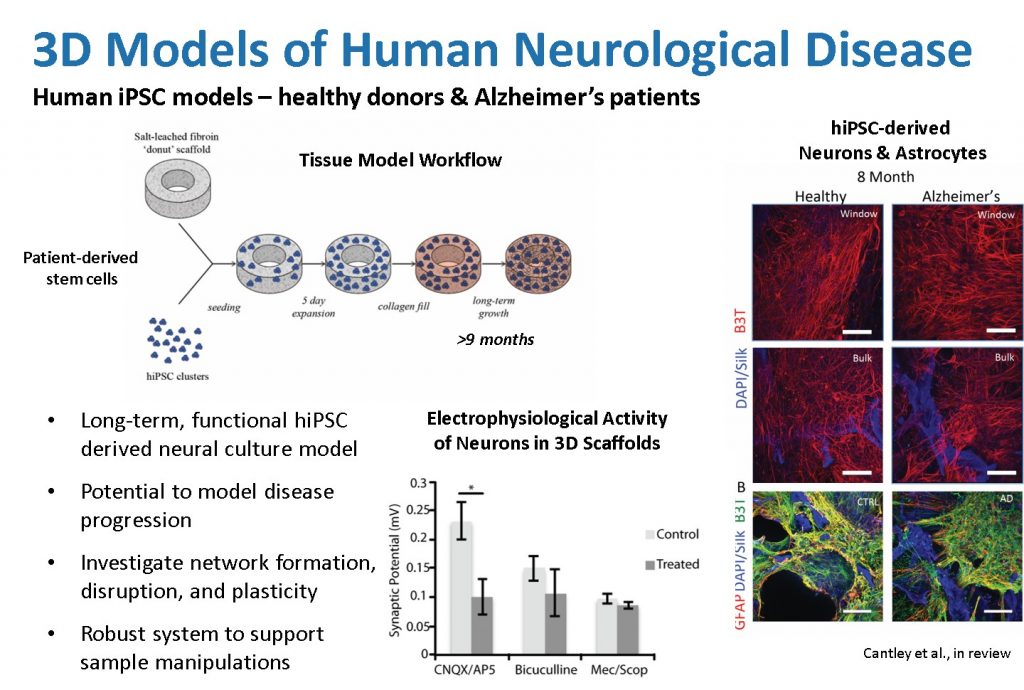
3D human neural network tissue model systems can support the attachment, growth, and differentiation of neurons derived from induced pluripotent stem cells (hiPSCs) from both a healthy donor and a patient diagnosed with Alzheimer’s Disease. These models support cell survival and long-term growth with cultures producing beta-III-tubulin (B3T+ neuronal) for at least 8 months, as well as glial fibrillary acidic protein (GFAP+ astrocytic) cells. This, combined with gene expression indicative of a diverse population of neurons including both excitatory (glutamatergic; GRIN1, GRIA1) and inhibitory (GABAergic; GABBR1) neurons, electrophysiological data using specific receptor antagonists (CNQX/AP5 and Bicuculline, respectively), and observations of beta-amyloid plaques in long-term tissue cultures, demonstrates the relevance of the 3D brain tissue systems to represent many of the phenotypic and genotypic features characteristic of Alzheimer’s disease. These models serve as the basis for future study of mechanisms of neurological disease progression and evaluation of pharmaceutical interventions.