The gut-immuno-brain-axis plays a critical, yet poorly defined role in the etiology of neurodegenerative and psychiatric disorders. Changes in the gut microbiome composition and metabolism are key components in onset and progression of Parkinson’s (PD) and Alzheimer’s disease (AD). Furthermore, severe gastrointestinal symptoms are a common comorbidity in patients with autism spectrum disorder (ASD). An amalgam of changes in gut-brain disease hallmarks, including changes in gut permeability, production of neuromodulatory compounds, and activation of the enteric nervous system (ENS), vagal nerves, peripheral immune system, microglia and astrocytes may lead to defects in neural network development, function, atrophy, and ultimately central nervous system disease.
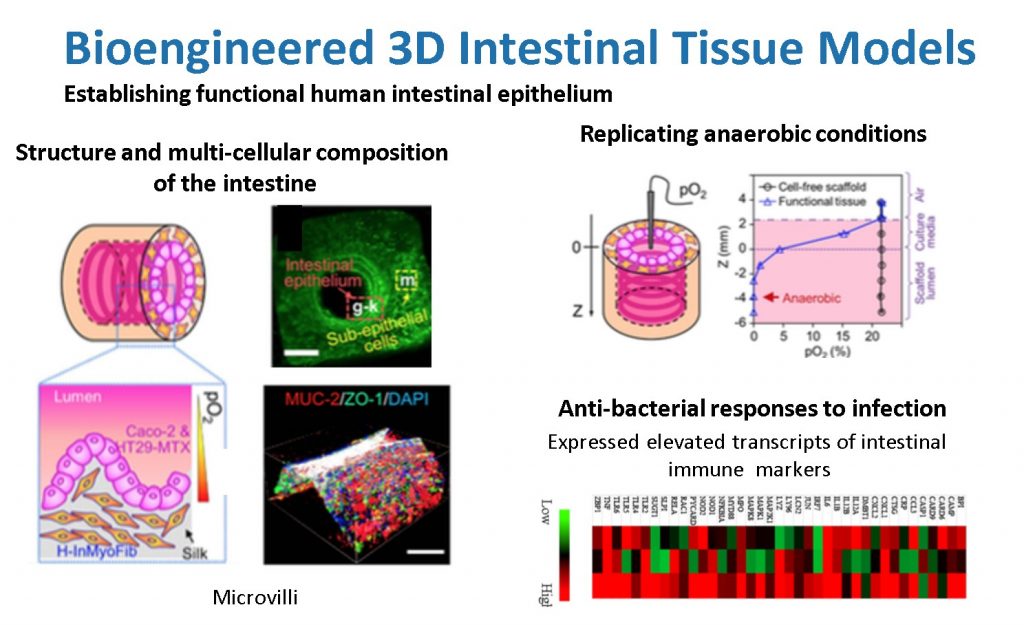
Our novel 3D in vitro tissue models of human intestine mimic both the structure and function of native intestine. The resulting in vitro intestinal models properly represent the characteristic functions of human intestine, are innervated, and demonstrate physiological outputs (e.g., digestive enzyme and mucous secretion, support of low or anaerobic oxygen level, and inflammatory response to infections). We have successfully incorporated human intestinal primary epithelial cells, primary intestinal enteroids and colonoids into these 3D systems, and we have successfully tracked bacterial infections. Sustained cultivation of the intestine system for months, together with the scalable and integrative nature of the tissue engineering techniques, provide novel systems with which to gain new insight into human intestinal infections.
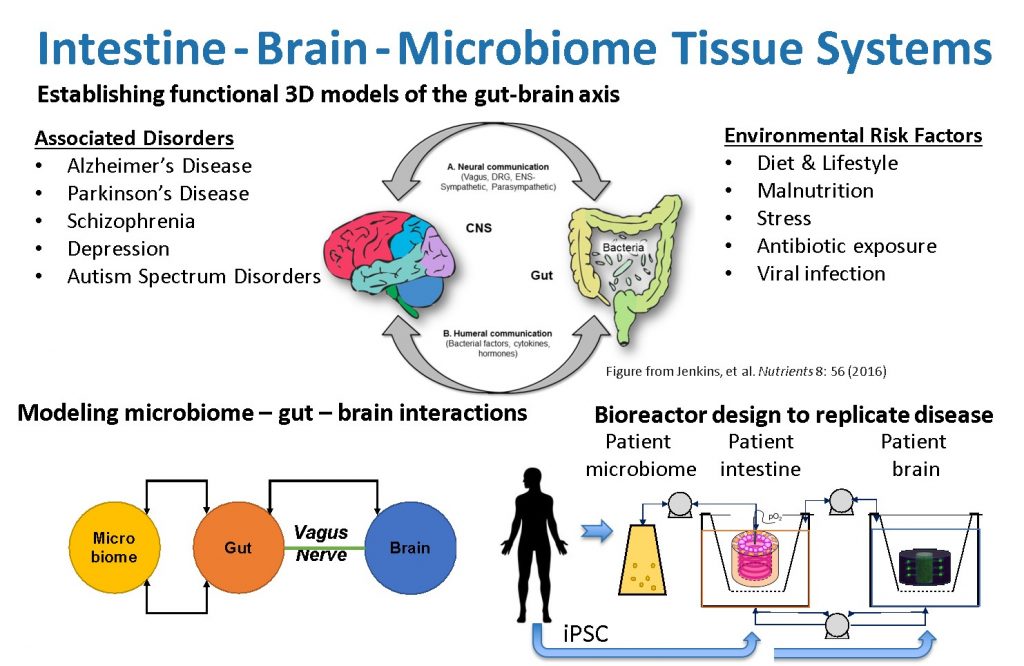
By linking our current 3D brain and gut tissue systems, both of which may be cultivated for weeks to months and demonstrate structural and functional relevance for human conditions, we can assess inter-tissue signaling. Our unique, universal three-dimensional in vitro gut-brain organ experimental platform will incorporate patient microbiome and iPSC cells to elucidate fundamental disease mechanisms. This will establish a direct route of communication between the gut microbiota and the brain in a well-controlled, human cell-derived in vitro system. Bioengineering, microfluidics and stem cell technology will replicate the heterocellular and multi-organ composition, architecture, connectivity, and functionality of the gut-brain-axis. We will identify the network of microbomes, molecular pathways and cellular interactions that cause Parkinson’s disease. Future studies will use these approaches to develop gut-brain models to unravel the mechanisms of other neurological disorders, allow identification of key molecular mediators, and offer novel targets for disease management.