Lipid Peroxidation
Glutathione (GSH) protects erythrocyte membranes from the toxic effects of heavy metals by forming complexes.22 After the complex forms, the concentration of free GSH decreases. In a study produced by Sugawara et al,23 workers who were exposed to lead were shown to have only 69% of the glutathione compared to the control group. Furthermore, other antioxidants such as Superoxide Dismutase (SOD) and catalase also decreased in the presence of lead, and workers that had been exposed to lead only had 42.8% and 57.0%, respectively, of the enzyme activity compared to the control group.23
Like GSH, SOD and catalase work to protect the cellular membrane against harmful oxygen species such as superoxides and peroxide. Since these enzymes, as well as glutathione peroxidase which oxidizes GSH, form complexes with the lead, they are unable to perform their normal antioxidant function when in the presence of lead. As a result, the cellular membrane is susceptible to lipoperoxidation.
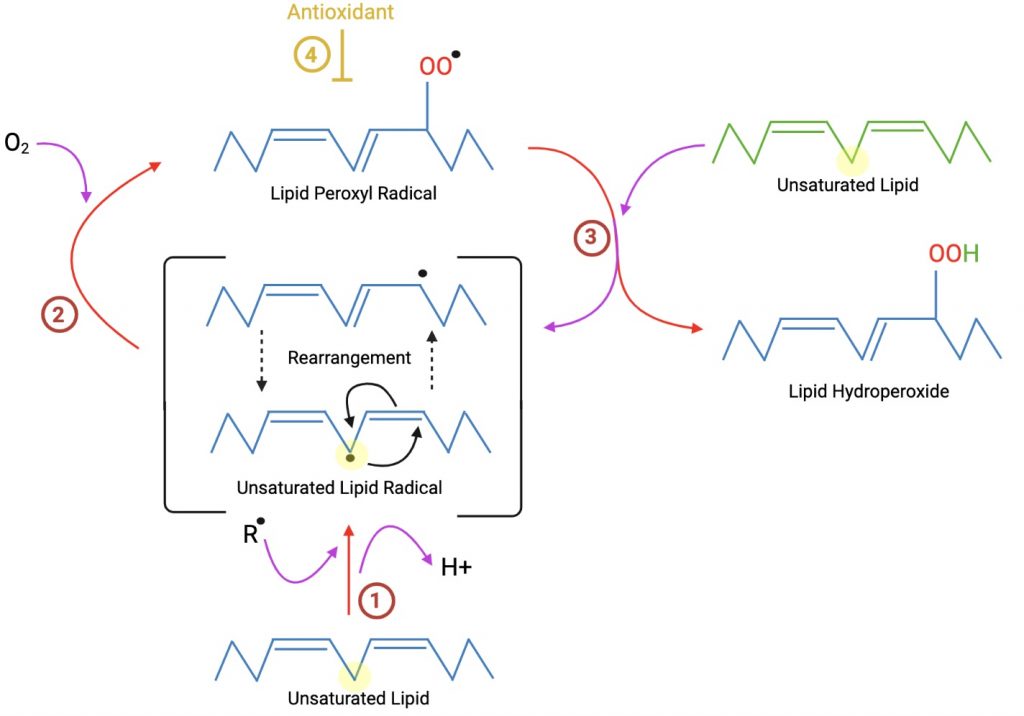
Sugawara et al23 observed increased levels of hemolysis in workers who had been exposed to lead, caused by lead’s peroxidation of the erythrocyte membranes. This phenomenon is intensified when lead associated ROS are also interacting with platelets, leucocytes, or both. The observed increase in hemolysis is caused by the white blood cells’ and platelets’ mitochondria reacting to the oxidative stress by producing more ROS.25 These observations were made only when lead was also in the presence of hemoglobin, and in its absence, the lead seemed to have a lesser effect.23 GSH usually maintains the cysteine residues of hemoglobin and other proteins. In lead-induced anemia, Heinz bodies were found within the red blood cells, suggesting oxidative damage to the hemoglobin itself by lead, which may explain why hemoglobin must be present for the hemolysis to be significantly increased from normal.23
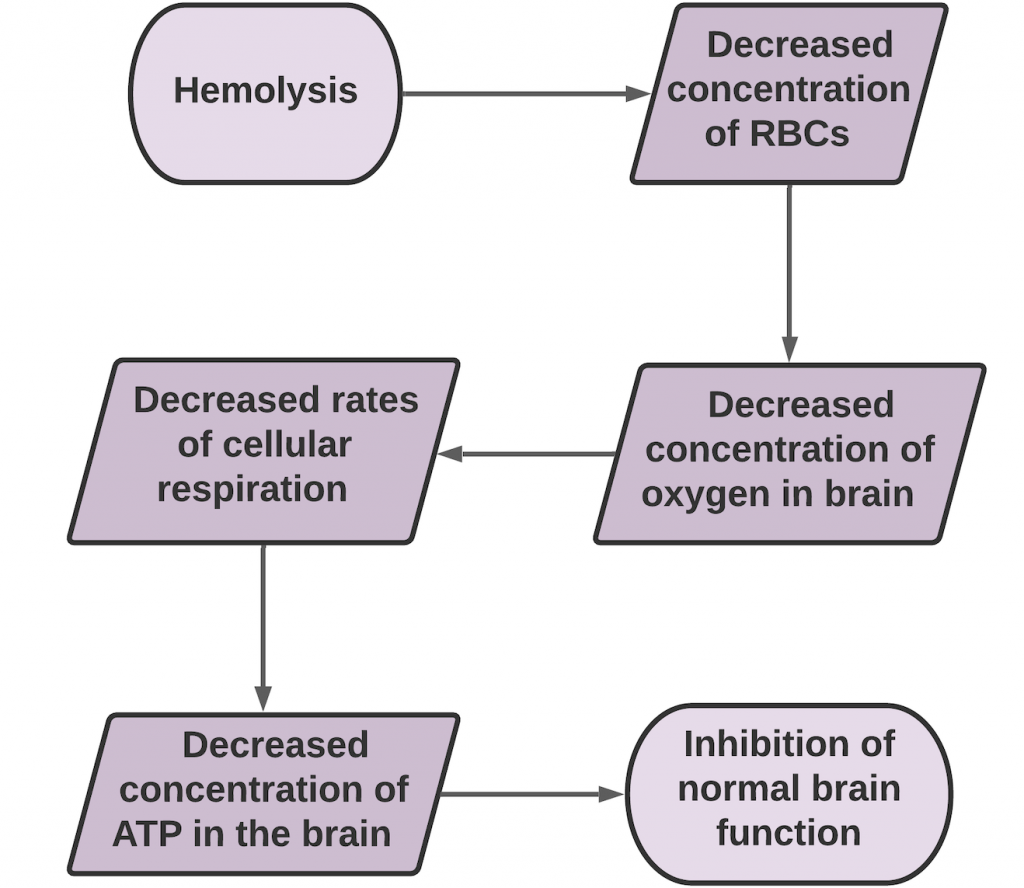
Ultimately, it is the increased production of intermediate oxidizing agents such as hydrogen peroxide and superoxide radicals as well as the decreased activity of antioxidants SOD, catalase, and glutathione peroxidase that cause the membrane lipid peroxidation.23 In order to mitigate the lipoperoxidation, RBCs must attempt to maintain homeostasis from either increased utilization of GSH and NADPH, or the flux of glucose through the pentose phosphate pathway (PPP) must be inhibited.23 These pathways, however, cannot fully combat the negative effects of ROS from lead, and hemolysis and early erythrocyte death are likely after lead exposure. With a decreased concentration of erythrocytes, the brain’s ability to get enough oxygen is in jeopardy, which can inhibit normal brain function.
Recent Comments