ROS Generation and ALA
Lead’s inhibition of δ-Aminolevulinic Acid Dehydratase (ALAD) causes the heme precursor, δ-aminolevulinic acid (ALA), to accumulate.26
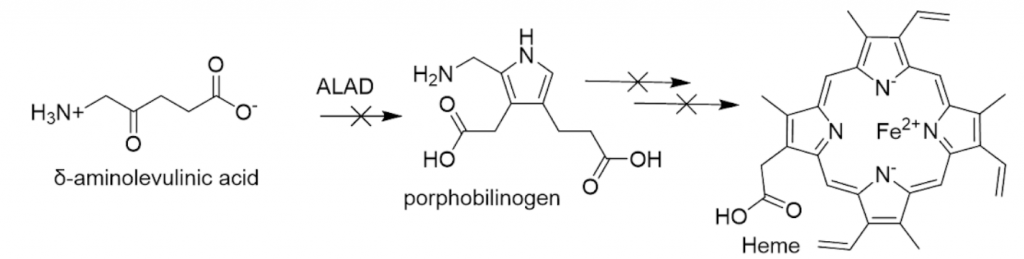
Inhibition occurs because lead displaces zinc in the enzyme’s metal binding site (see ALAD Inhibition for detailed description of ALAD inhibition and the enzyme’s function).27 ALA has been linked to generation of ROS. Heightened levels of Superoxide Dismutase (SOD) and glutathione peroxidase (GSH-Px) are found in individuals who have lead-inhibited ALAD.28 Notably, this outcome is also seen in patients with acute intermittent porphyria (AIP). This is a genetic disease with neuropsychiatric symptoms, characterized by enzyme deficiencies in the heme synthesis pathway and resultant buildup of ALA.29The abnormal elevation of these antioxidant defense enzymes in both conditions indicates that their common trait -ALA accumulation- is linked to build-up of ROS.
Free radicals may be generated through the autoxidation of ALA, as enolization and autoxidation can occur at pH 7.0-8.0.29
The oxidation of ALA to 4,5-dioxovaleric acid (DOVA) produces ROS, namely hydroxyl radicals, superoxide, and hydrogen peroxide.31
The formation of hydroxyl radicals from superoxide and hydrogen peroxide is iron catalyzed.29Production of superoxide is supported by the parallel reduction of ferricytochrome c; additionally, spin-trapping experiments (a method used to detect the presence of short-lived free radicals) support formation of a hydroxyl and carbon-centered radical.32 The elevated ALA levels that result from inhibition of ALAD thus can increase the concentration of ROS present. Also, ALA oxidation is associated with the release of iron from ferritin, furthering oxidative stress;33 as aforementioned, free iron can catalyze the conversion of hydrogen peroxide to free radicals. In regard to genotoxicity, ALA’s oxidation has been reported to cause DNA single-strand breaks and the product of its oxidation, DOVA, has been reported to alkylate DNA.26,34
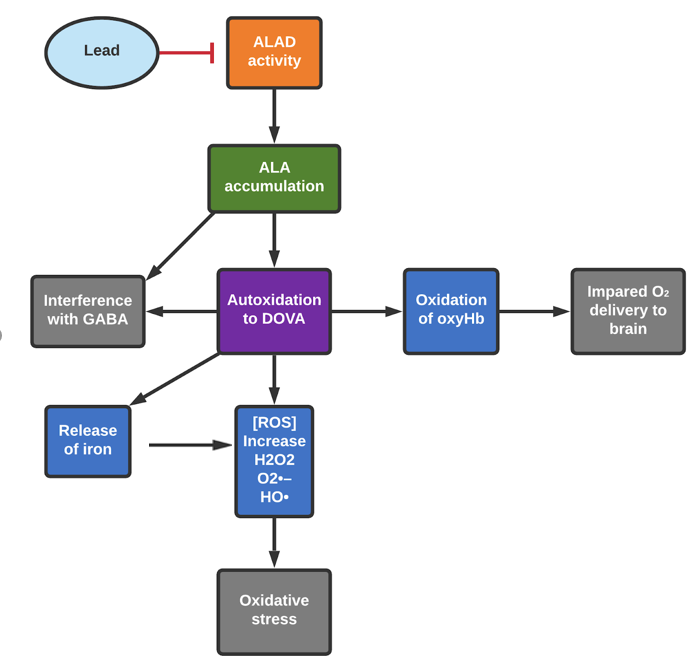
The interaction between ALA and oxyhemoglobin (oxyHb) is also linked to formation of ROS, exacerbating oxidative damage. The enol tautomer of ALA can induce autoxidation of oxyHb to methemoglobin (metHb).29MetHb contains iron in the +3 state rather than +2, which prevents it from binding to oxygen.35 The ALA-enol and oxyHb each donate an electron to molecular oxygen, producing metHb, ALA radical, and H2O2.32 The superoxide and H2O2 produce highly reactive hydroxyl radicals.32
MetHb production in itself is problematic, as metHb cannot effectively deliver oxygen, reducing blood oxygen levels.35 Furthermore, hemoglobin oxidation by free radicals as produced in these pathways causes hemolysis (hemolytic anemia). Overall, oxidative processes associated with ALA decrease the efficiency of oxygen delivery. This compounds the oxygen delivery issues resulting from interrupted heme (and thus hemoglobin) synthesis (see ALAD Inhibition). The central nervous system requires high levels of oxygen to function properly, as the brain relies on aerobic metabolic processes for energy, so impaired oxygen delivery is especially detrimental to central nervous system function.
ALA has the ability to cross the blood-brain barrier and can accumulate in nervous tissue.29 This is significant, as the blood-brain barrier regulates what comes into contact with the central nervous system; ALA and its associated ROS production during autooxidation can directly interfere with processes necessary for nervous system function. Free radicals can impair selective high-affinity reuptake of the neurotransmitter gamma-aminobutyric acid (GABA) at the synaptosomal membrane.36 The ROS associated with ALA oxidation also interferes with GABA receptor function.33 It has been observed that treatment with ALA decreases GABAergic receptor density and that DOVA interacts with GABA receptors.33 As GABA is the primary inhibitory neurotransmitter, the reduction of its action could increase the activity of excitatory pathways, which can lead to seizures and convulsions.33 (see ALAD Inhibition for further information about the relationship between ALAD inhibition and GABA). Radicals associated with ALA oxidation contribute to lesions in the habenular complex (region of brain that influences stress response, mood, behavior, and learning), which could present as behavioral symptoms.33
Recent Comments