Luciferase Mechanism
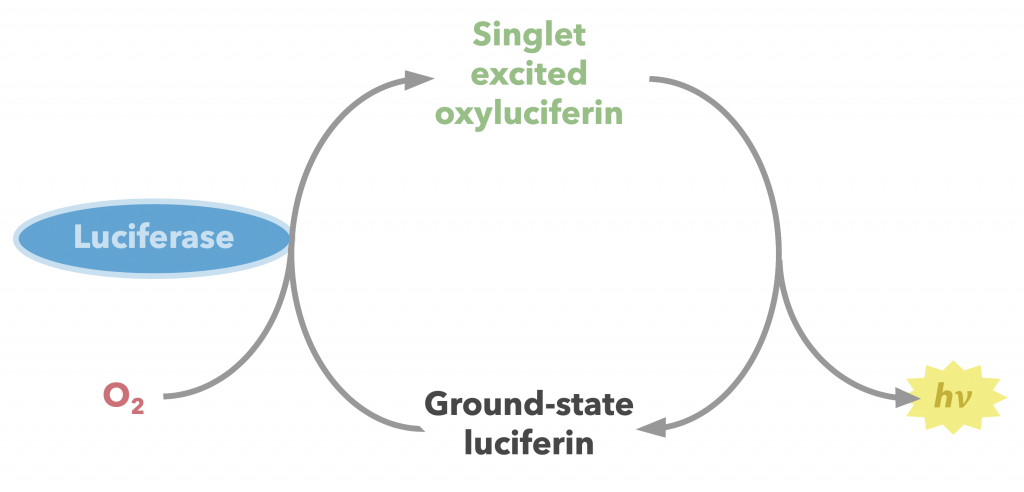
The enzyme firefly luciferase (referred to herein as simply luciferase) catalyzes the conversion of firefly luciferin into an oxidized, excited form, which then produces fluorescence as it relaxes (Figure 24) (22). This florescence is measurable, making this substrate-enzyme combination a valuable quantitative tool in a wide array of biochemical assays including but hardly limited to the opioid receptor binding assay described earlier. Other useful applications include quantification of transcription and assessment of cell viability. A step-by-step breakdown of the mechanism is described below.
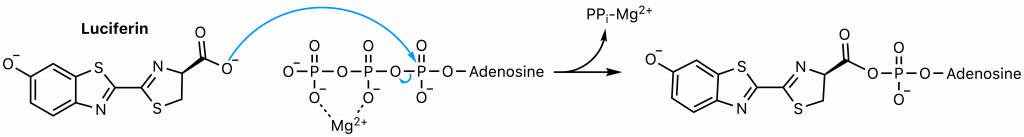
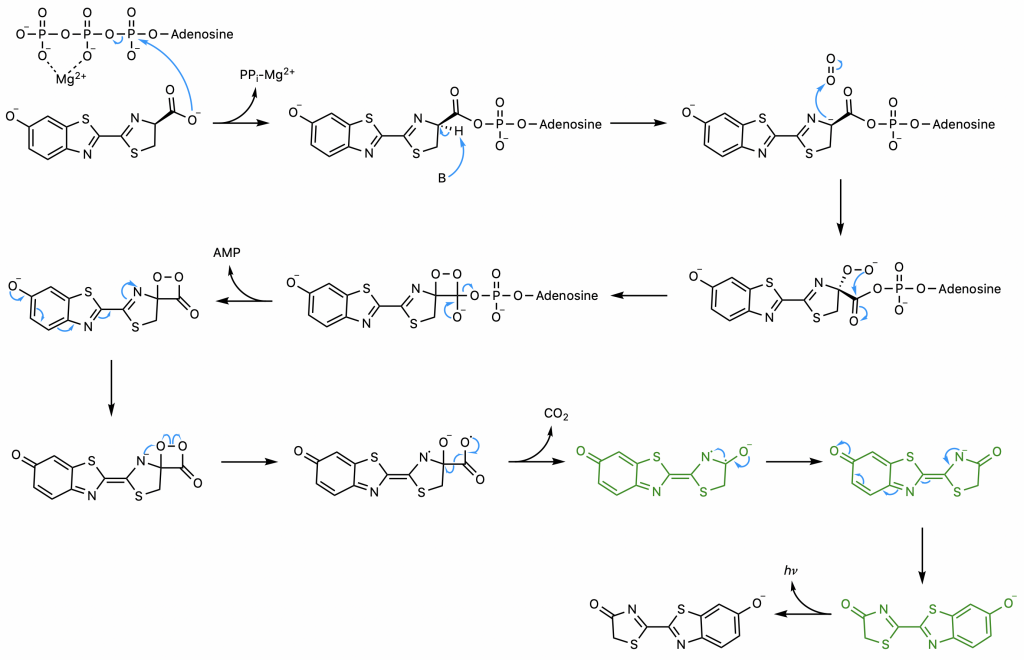
First, luciferase catalyzes the SN2 reaction of luciferin and ATP, the adenylation step (23). The carboxyl group of luciferin attacks the highly electrophilic alpha phosphorous of ATP, displacing pyrophosphate, a superb leaving group. The common strategy of magnesium coordination is employed to enhance the electrophilicity of the alpha phosphorus and stabilize the resultant free pyrophosphate.
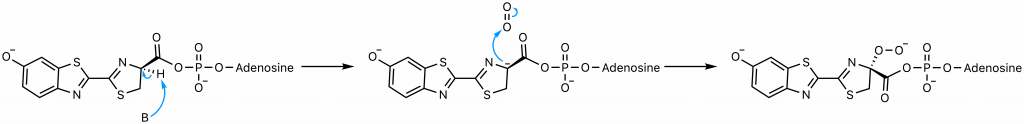
The presence of the AMP substantially increases the acidity of the proton on carbon 4 of the thiazoline ring by delocalizing the negative charge that would result from its abstraction (23). This abstraction is exactly what occurs; the enzyme catalyzes a deprotonation to yield a carbanion at carbon 4 stabilized not only by the AMP but also by the conjugated system to its left. A hydroperoxide then forms as the carbanion attacks molecular oxygen, the oxidative step of the reaction.

The hydroperoxide attacks the carbonyl carbon to form a 4-membered ring, a process favorable due to the end result of this nucleophilic acyl substitution, the loss of the good leaving group AMP (23). The resultant alpha-peroxy lactone is a high-energy intermediate called a dioxetanone, a conserved motif across several chemiluminescent systems (24).
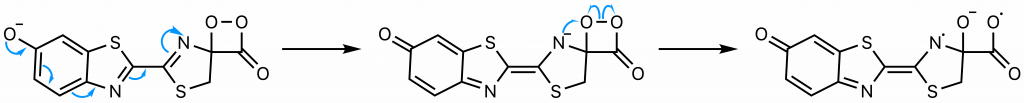
The sequence of events comprising the decomposition of the dioxetanone are the subject of some debate (22). One proposed set of steps (22) will be presented here . Through resonance, the rightmost nitrogen on the dioxetanone compound has anionic character. As an anion, it participates in a radical reaction in which the high-energy peroxide bond undergoes homolytic cleavage and one electron from the nitrogen is donated to the oxygen on carbon 4, ultimately yielding a diradical.
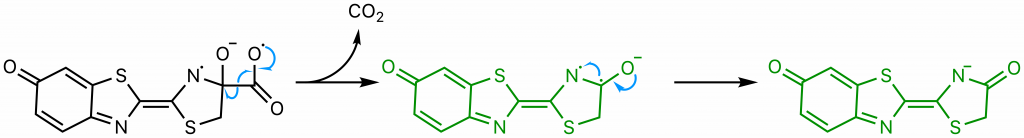
The diradical is very unstable, and undergoes spontaneous decarboxylation in another radical reaction to form singlet excited oxyluciferin (green) (23). The two unpaired electrons then combine. Although only the keto version of oxyluciferin is shown here, the enol and enloate can also form.
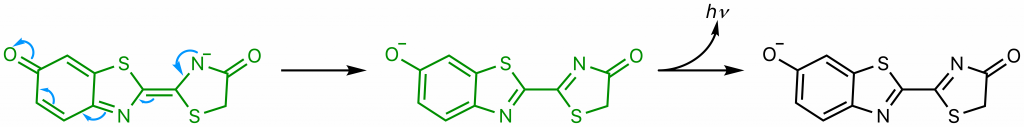
From here, resonance restores oxyanionic oxyluciferin, and the excited form (whether it be keto, enol, or enolate) returns to ground state, causing the emission of a photon that accounts for the observed luminescence (24). Overall, the reaction catalyzed by firefly luciferase is similar to many other chemiluminescent and bioluminescent processes, falling under the category of chemically initiated electron exchange luminescence.
November 9, 2019 at 11:18 pm
It seems like this page would almost work better if it were before the opioid receptor binding assay page because it explains how the enzyme works. Then you could say how it is used to determine how opioids work in the human body.
December 14, 2019 at 11:36 pm
That definitely makes sense. To be honest, understanding this mechanism isn’t super essential to understanding our project, but the assay is a little bit more so. We wanted to present the assay first and just have this as an optional page people could look at in case they were curious about how luciferase works.
November 10, 2019 at 5:56 pm
It’s very difficult to explain each detail in a mechanism, but you were successful in being as specific as possible. I am just confused how this mechanism plays a role in the rest of your website. It is a valuable quantitative tool in such opioid binding assays but maybe elaborate more on why is is useful, and less on the actual mechanism.
December 14, 2019 at 11:37 pm
Added some more examples of how it’s applied. Thanks!