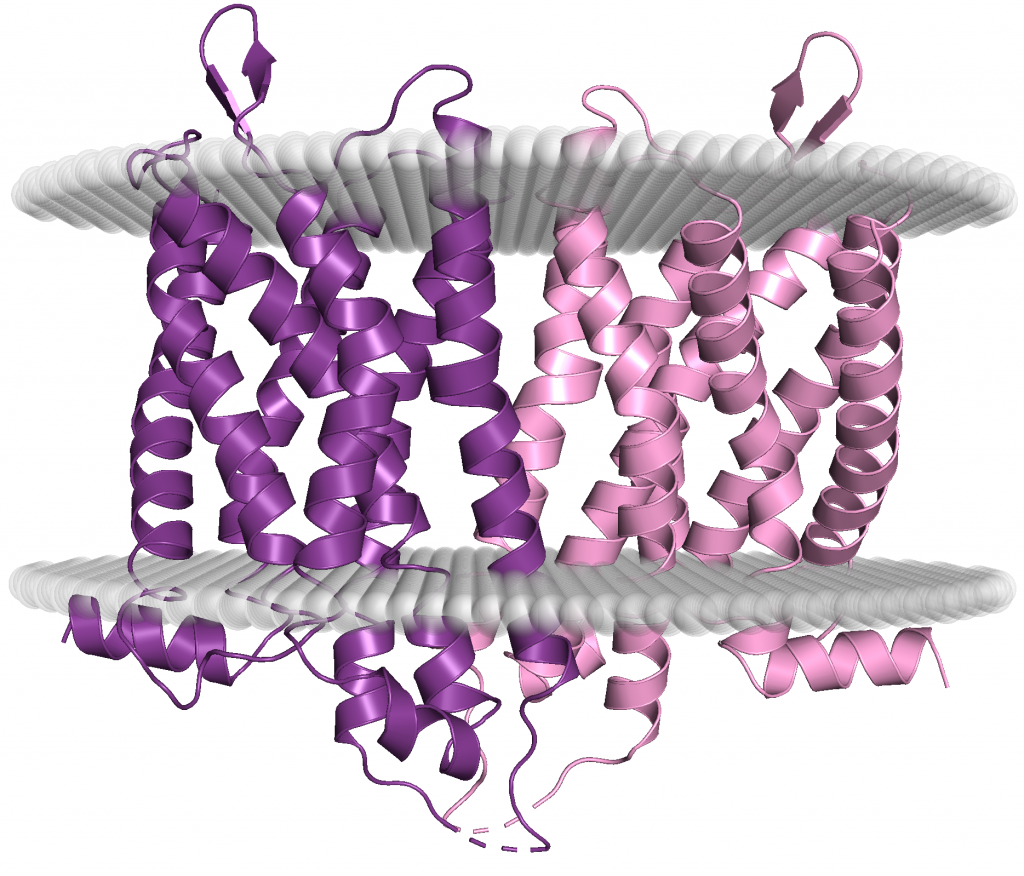
µ Receptors: Like all opioid receptors, µ receptors are GPCRs. This means their structure is dominated by seven transmembrane alpha-helices and the attached G-proteins (not pictured in the figure above). µ receptors specifically bind to the endorphin class of endogenous opioid peptides. They are distributed in the brain with very strong levels in the cerebral cortex as well as throughout the spinal cord and peripheral nervous system (14).
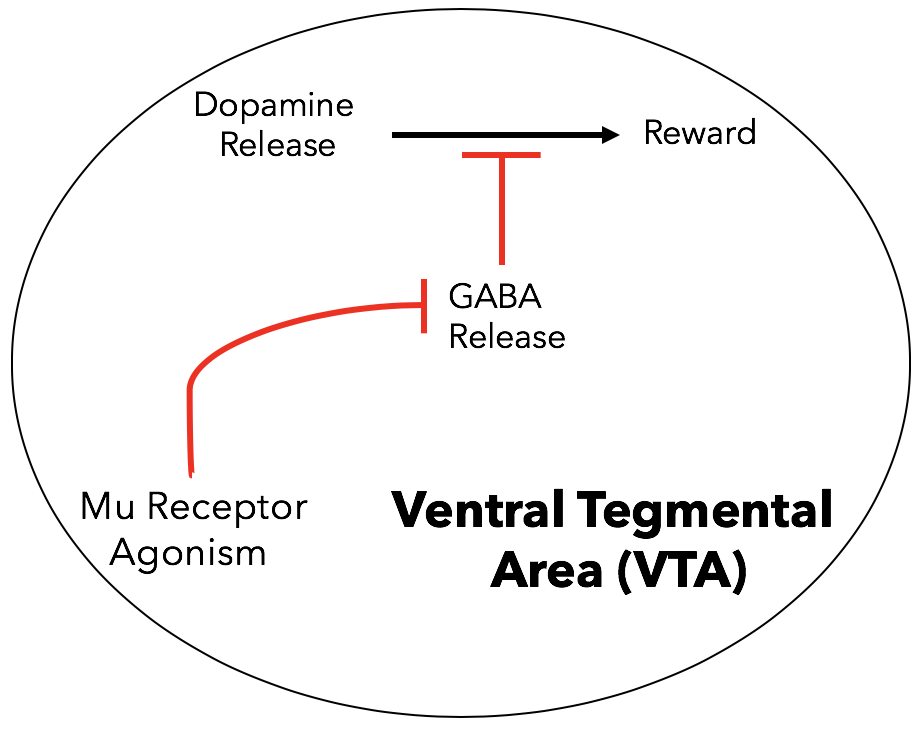
Mechanism of euphoria: What separates µ receptors from other opioid receptors is the secondary effects their activation has on the body. When agonists bind µ receptors in the ventral tegmental area of the brain (VTA), an important structure in the brain that mediates reward conditioning, the release of the neurotransmitter GABA is inhibited (15).
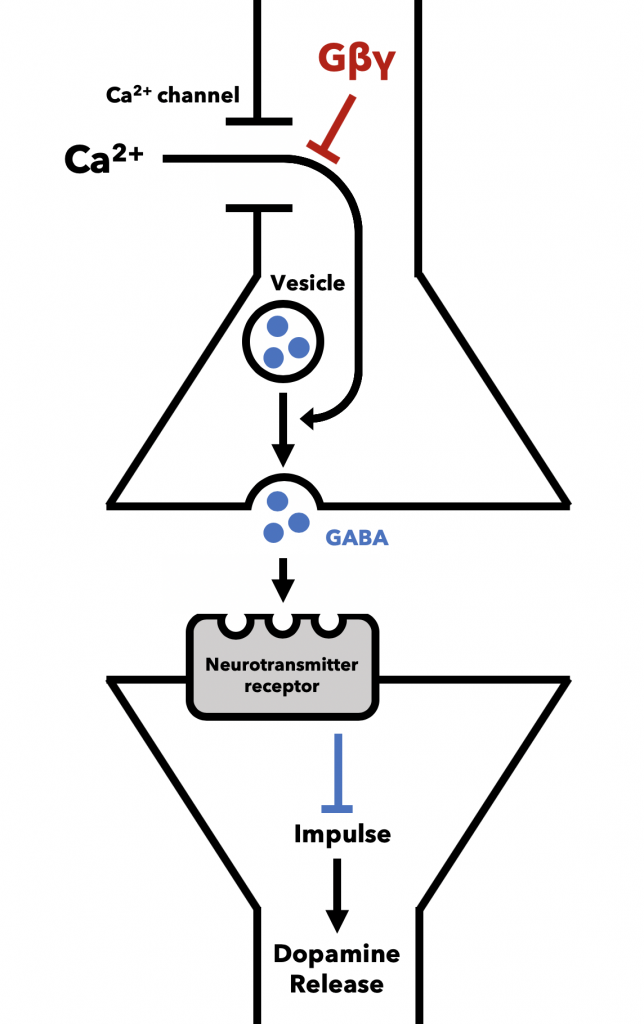
GABA is an inhibitory neurotransmitter, meaning when it binds its receptors on the postsynaptic membrane it inhibits the generation of a nerve impulse (Figure 12). In the VTA, GABA-releasing neurons are positioned to prevent nerve impulses in dopamine-releasing neurons, which yield feelings of reward like euphoria. These GABA-releasing neurons express µ opioid receptors (13). When bound, they release a Gβγ subunit that blocks calcium channels, ultimately preventing the release of GABA (see general opioid signaling). To summarize, in a sort of biological double-negative, µ opioid agonism in the VTA inhibits the inhibitory action of GABA on dopamine-releasing neurons, resulting in promotion of dopamine release to other neurons. Dopamine release causes the euphoric feeling, or “high,” experienced during opioid use.
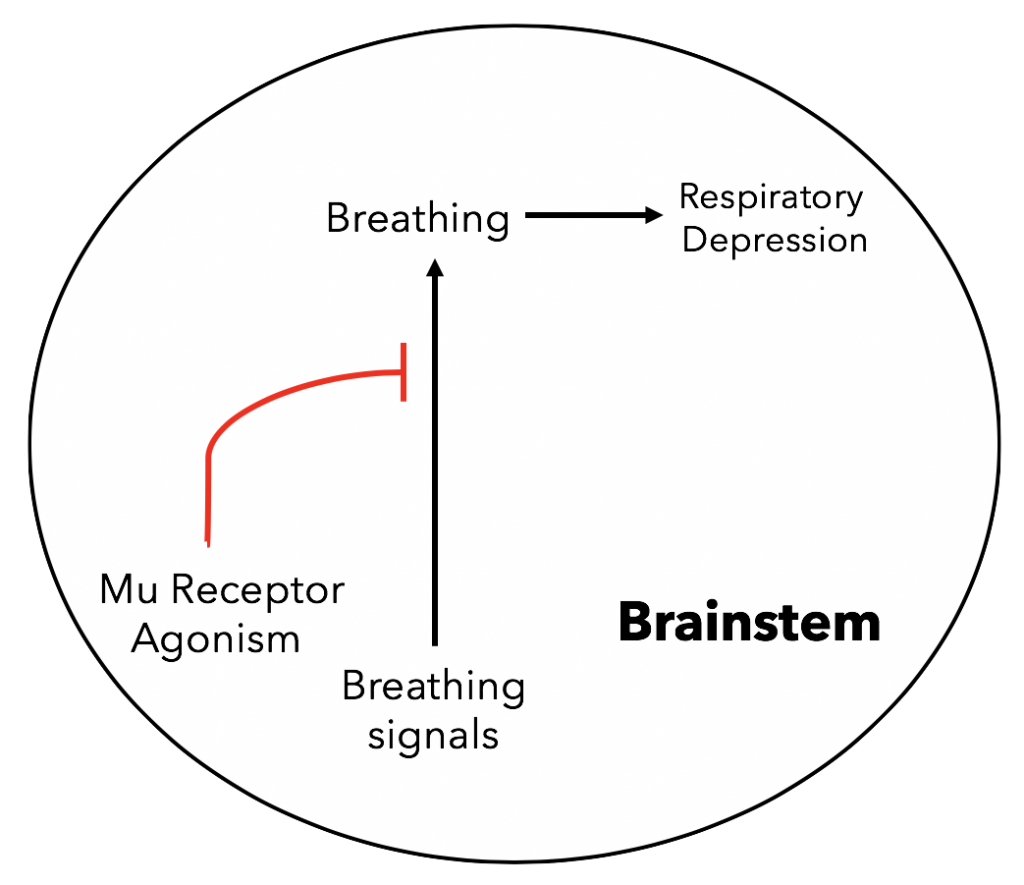
Mechanism of overdose: As for why taking excessive amounts is so dangerous, the actual cause of death for opioid overdose victims is respiratory depression. This occurs when µ agonist bind to µ receptors in the brain leading to the inhibition of brainstem neurons since as described earlier opioid signaling is inherently inhibitory (15). This is important since the brainstem controls respiratory rhythms and though preventing these neurons from firing eventually these breathing signals stop firing leading to the cessation of breathing or respiratory depression. This effect is the cause of opioid overdose and the majority of deaths in the opioid epidemic.
Overview: Euphoria and respiratory depression are the two most prominent side-effects of µ agonists. The euphoric effects of µ agonists stem from the inhibition of the main inhibitory neurotransmitter GABA in the VTA, a structure involved in the reward section of the brain. Meanwhile the respiratory effects are mediated though µ agonists inhibiting the neurons of the brainstem causing breathing signals to stop firing and the cessation of breathing. These two side effects combine to make µ agonists not only highly addictive drugs because of the euphoric effects but also highly lethal drugs because of its depressive effects on breathing.
I appreciate that you explain why the mu agonists are so addictive instead of letting the reader infer why. I am a little confused why people feel euphoric when all the dopamine receptors are blocked. I understand there is huge dopamine being released, but what is it being received by?
So the dopamine receptors being blocked is not important but rather the fact that one of the major elements inhibiting dopamine, GABA, is being removed when Mu agonists bind at this area. This produces euphoria. Additionally all neurotransmitters, such as dopamine, are sent to other neurons in order to send signals around the brain. I have updated the section accordingly to better explain this.
I think it would be good to give a little of the physiology behind addiction- it’s stated that dopamine increases, but talking about the changes in neuronal signaling pathways due to repeated use/excess dopamine conditions would help to concrete the topic for the reader and better explain why mu opioid drugs are so addictive as opposed to others/why the dopamine factor is so important.
While the physiology behind addiction is a very interesting research topic the main goal of this project is to explain why peptides can be used to develop nonaddictive pain medication. As a result we wanted to stick to the biochemistry and general pathways of addiction and opioid function and not go into physiology to give the background for the research we talk about later.
Explain what the VTA is/does more, and make the summary paragraph separate to highlight the take-away points.
I feel like every concept is explored thoroughly and made clear. However, I felt that in your earlier pages you mentioned how dangerous opioids were yet on this page you only devote two sentences to detailing how they can be lethal. Maybe a small paragraph or a bit more explanation on how they are lethal could help.