Non-addictive orally-active κ opioid agonist for the treatment of peripheral pain in rats (19)
Introduction: In this paper, Beck et al. from the Medical University of South Carolina expand on their group’s most promising peripherally restricted κ agonist peptide, JT09, that was first discovered in their lab six years prior. This study delves deeper into investigating the ability of this peptide to relieve pain and avoid unwanted side effects. In rat models, JT09 was assessed for depressant and sedative effects, reward properties, and addiction liability.
Background: Most opioid drugs are µ agonists with potent analgesic effects; however, they also introduce dangerous side effects such as euphoria, addiction, and tolerance. This has lead to a rising interest in developing κ agonists, which have none of the side effects listed above but relieve pain with similar effectiveness. Early κ agonists, however, came with their own side effects. The ability to cross the blood brain barrier and enter the central nervous system (CNS) resulted in depressive and sedative effects. Therefore, if a compound could be developed to target κ receptors without entering the CNS, scientists believed that most, if not all, of the unwanted side effects currently associated with opioid based analgesics could be avoided. The result was peptidic compounds designed as peripherally restricted κ agonists.
CR665 and the development of JT09: A company called Cara therapeutics developed a peptide known as CR665, which was a peripherally restricted κ agonist (meaning it does not reach the CNS) showing great promise for pain relief. However, this peptide was not orally available, thus limiting its potential therapeutic applications. In 2013, Hughes et al. developed an 18-member library of CR665 derivatives, with the intention of improving oral availability (18). Five of these peptides showed great promise in their rat-based assays, as well as their cell-based binding experiment (Figure 27). Of these five peptides, JT09 was selected for further development due to its particular modification and ease of synthesis.
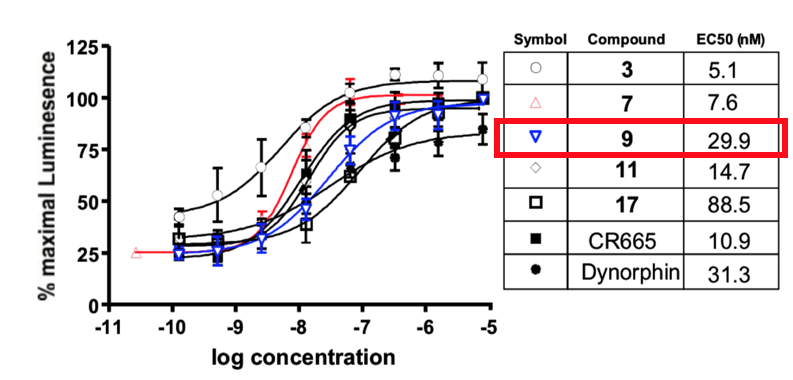
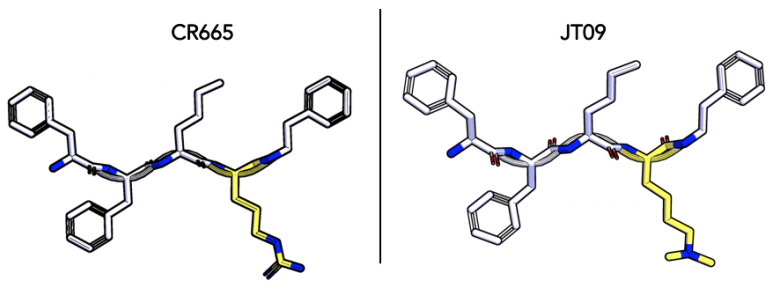
JT09: In a paper published in the European Journal of Pharmacology in 2019, JT09 was subjected to further testing to determine its efficacy and ability to avoid unwanted side effects. JT09 is structurally similar to CR665, with the only difference being the fourth residue. The D-arginine of CR665 was replaced with a modified D-lysine residue, a strategy that has previously shown to improve the oral availability of compounds used to treat psychosis, stroke and Neuropathic pain (18). The new peptide has an EC50 of 29.9 nM, is selective for the κ receptor over other receptor types by >33,400 fold, and has a peripheral selectivity of >938 fold. JT09 was also shown to have oral availability, making it a very promising drug candidate.
Rat-based testing: Their most recent paper used rat models to assess the efficacy and side effect profile of JT09.
Acetic acid-induced writhing model. The potency of JT09 in the treatment of peripherally mediated pain was assessed using the acetic acid induced writhing assay. In this assay, rats were treated with either JT09 or a positive or negative control, then injected with acetic acid. After injection, rats were observed every 20 seconds for 20 minutes and scored based on whether or not they were writhing at the time of observation. The percentage of time spent writhing was calculated and compared.
Hot plate model. Next, JT09 was assessed for the treatment of centrally mediated pain. Since the goal of this compound was to avoid crossing the blood brain barrier (to avoid dysphoria), the intent was that JT09 would not have analgesic efficacy in the central nervous system. To perform this test, rats were again treated with the compound of interest, then place on hotplate. The time until the rat responded to the stimuli (known as response latency) was recorded and reported as a percent of the maximum possible effect.
Self administration model. To test for addictive potential, rats were studied in a self administration model. In this test, rats were trained to press a lever for intravenous drug delivery. After a day of receiving a sucrose pellet for training, rats were then given an intravenous dose of JT09 in response to lever pushing for 5 days. After those 5 days, JT09 was replaced with cocaine as a positive control for reward processing.
Forced swim assay. To assess depressive effects, rats were placed in a 30ºC chamber of water without an escape. The time that the rat spent immobile duration of the trial, was taken as a measure for depressive like effects.
Locomotor open field test. Lastly, to test for sedation , rats were tested in the locomotor open field test. In this assay, rats were placed in an automated activity chamber for 30-minutes, and the distance traveled in that time was recorded.
Results: Analgesic efficacy. The assays described above were used to assess the analgesic potency, risk for addiction and depressive and sedative effects of their leading peptide based κ opioid agonist, JT09. The results of these tests showed that JT09 was statistically indistinguishable from morphine in its ability to mediate peripheral pain in the acetic acid writhing assay. The peptide did not show analgesic activity in the hot plate model, meaning that it did not alleviate centrally mediated pain. This result supports the claim that JT09 does not cross the blood brain barrier.
Results: Avoiding unwanted side effects. JT09 did not exhibit reward properties in the self administration model, which is interpreted as a reduced risk for addiction. Similarly, JT09 was statistically indistinguishable from the saline control in both the open field test and the forced swim assay, showing that the leading peptide did not show significant depressive or sedative effects.
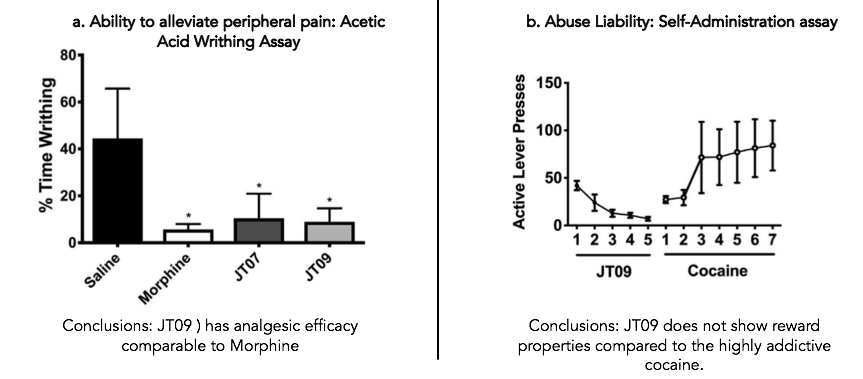
Conclusions: The data show that JT09 is orally active and peripherally restricted, meaning that it does not cross the blood brain barrier. It is comparable to morphine in its efficacy to alleviate peripheral pain, and does not exhibit reward properties or induce depressant or sedative effects.
I love how this page is broken up like an actual report. it makes reading the results and why it was done clear and concise. It is really good to know that scientists are trying to find ways to reduce addictive side affects to necessary pain management drugs! I am still a little confused on the hot plate test. were the scientists burning the rats and seeing whether or not they responded to the pain? or was it to test if the medication sedated them?
The hot plate model was used to to assess whether or not the drug relieved centrally mediated pain, meaning that they were looking to see if it crossed the blood brain barrier (the goal was that it didn’t!) . The locomotor open field test and the forced swim assay were what this group used to see if the drug caused any sedative or depressive effects.
I like this section because it shows real life application to the website’s focus, giving real facts to help your purpose. Maybe another title rather “Paper 1” and “Paper 2” may appear more interesting to click on.
Maybe just a few words taken from the title below, to catch the readers attention