Kappa Receptors
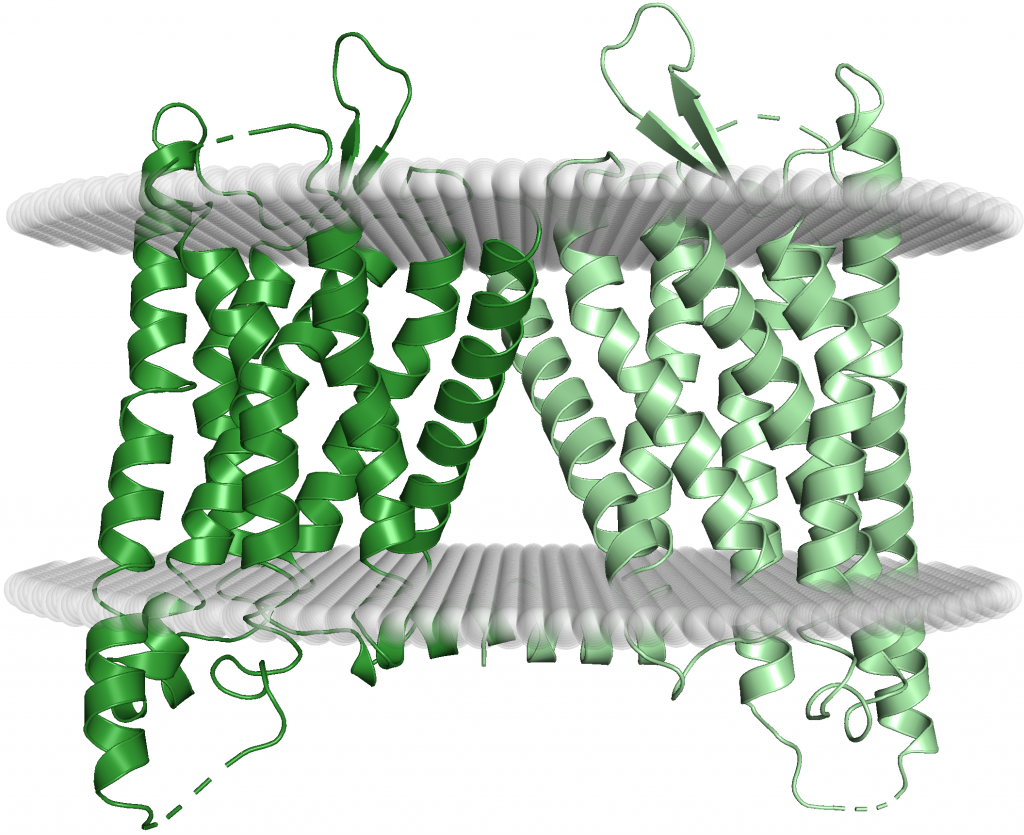
κ Receptors: Like µ receptors, κ opioid receptors are primarily made up of the seven transmembrane alpha-helices characteristic of GPCRs (Figure 14). The distribution of κ receptors is similar to that of µ receptors, albeit with a slightly higher concentration in the cortex and a slightly lower one in the midbrain (14). The main difference between κ and µ receptors are the side effects. When κ receptors are bound, they do not inhibit the neurotransmitter GABA and cause feelings of euphoria like µ receptors do. Rather, stimulation of κ receptors can cause strong feelings of dysphoria, a state of unease that often accompanies severe mental disorders, such as depression and bipolar disorder (effectively the opposite of euphoria). The severe dysphoric effects along with the fact that that mu opioids were developed first and work well in a hospital setting, as in the case of morphine, meant that κ agonists were not a main priority in drug development. The method by which κ opioid receptor activation causes dysphoria is poorly understood but in this section we explore two possible theories behind the mechanism of dysphoria of κ agonists.
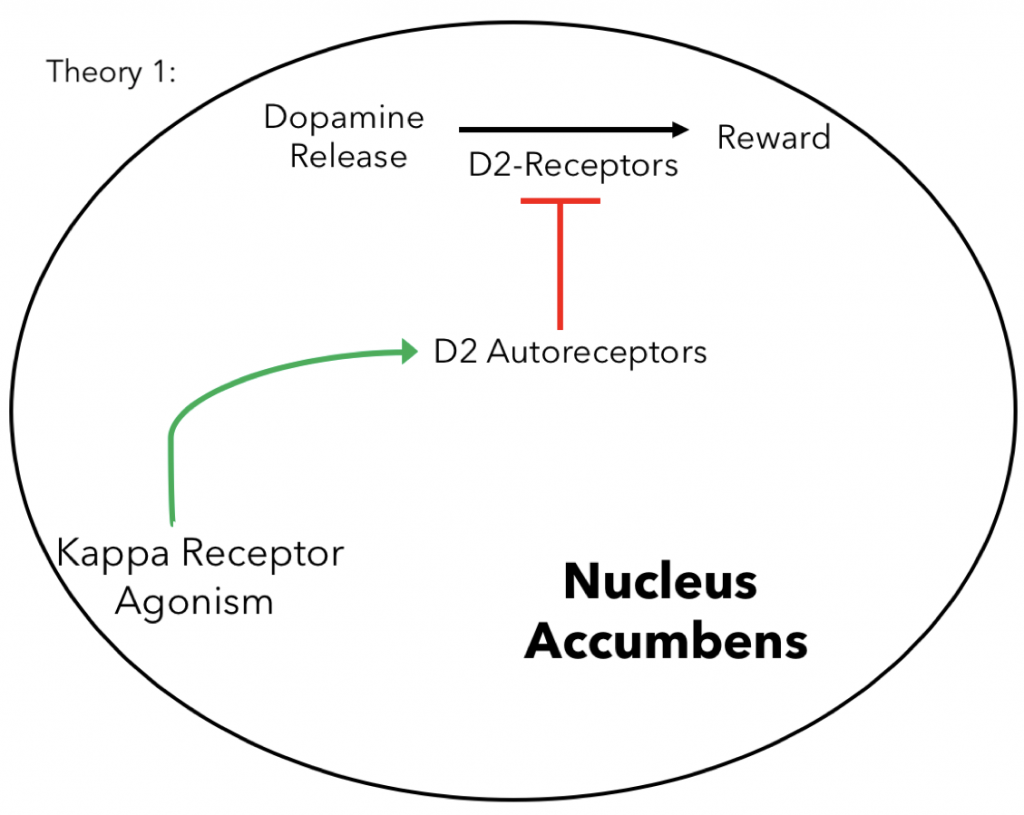
D2 Autoreceptor Theory: One theory behind why κ opioid receptor activation causes dysphoria has to do with autoreceptors specifically Dopamine-D2 receptors (D2-receptors) in nucleus accumbens, another brain structure involved in the reward system of the brain. Autoreceptors are receptors on the pre-synaptic membrane of neurons that when stimulated participate in negative feedback inhibition for a particular neurotransmitter. In the case of D2-receptors they inhibit dopamine. Recent research indicates that the coactivation of κ receptors with D2 receptors in the nucleus accumbens decrease the sensitivity of D2-receptors overtime increasing their activity (16,17). This is important since enhancing the activity of D2-receptors increases dopamine inhibition in the nucleus accumbens potentially leading to the dysphoric side effects of syntheic κ agonism.

MAPK Theory: However, other research suggests the dysphoric affects of κ receptors may not come from inhibition of dopamine in the nucleus accumbens but rather from alteration of mitogen-activated protein kinase (MAPK) signaling in the VTA (18). Briefly, MAPK signaling is a very important pathway in signal transduction since can greatly amplify extracellular stimuli. This process begins when a receptor tyrosine kinase (distinct from the µ receptor) on the cell membrane is phosphorylated, catalyzing the phosphorylation of a series of other other intermediate molecules to amplify the signal. This can then be used to cause a series of intracellular changes, especially gene expression. This relates to the dysphoric effects of κ agonists since deletion of p38α, a protein involved in p38 MAPK signaling, was shown to reduce the adverse affects of κ activation independently of dopaminergic inhibition (18). This suggests that the dysphoric effects may be caused by alterations in gene expression. Narrowing this down to a specific gene is difficult since may gene are up and down regulated in MAPK signaling so more research is needed to find the specific genes involved. Regardless of the mechanism, dysphoria, like euphoria for µ receptors, is mediated exclusively by κ receptors localized in the central nervous system, specifically the VTA and other structures involved in the brain’s reward system.
Overview: While the precise mechanism of the dysphoric side effects of κ agonist remains unknown current theories suggest it is most likely mediated through the central nervous system, specifically D2 autoreceptors in the nucleus accumbens and MAPK signaling in the VTA. This is also true for the euphoric and respiratory depression effects µ agonists which are also mediated through the central nervous system. In the next section we discuss the potential of peptide therapeutics to provide pain relief while avoiding the central nervous system mediated effects of conventional κ and µ agonists.
November 8, 2019 at 5:43 pm
On both the mu and Kappa receptors, I am curious as to which drugs affect each receptor. If its possible, maybe provide examples of different drugs that affect them? are illegal drugs more prone to affect the kappa than mu? or is it about equal?
December 14, 2019 at 11:08 pm
So pain-relief drugs are made to bind very strongly to one specific receptors, either mu or kappa. Synthetic mu receptor agonist though make up all of the traditional opioids, such as morphine, oxycodone and heroin as covered in the answer section. However there are no synthetic kappa receptor binding drugs that are approved for human use. So to answer your question all conventional opioids bind to mu receptor.
November 10, 2019 at 5:24 pm
Dysphoria is a little confusing to me- kappa agonists are preferentially being researched, but the symptoms of dysphoria make it seem like a similarly non-ideal drug solution as instead of addiction and respiratory issues it decreases dopamine uptake. As it reads now, it seems like the desensitization of D2 receptors mean that the neurons have a harder time receiving dopamine, in which case it would be interesting to have a sentence or so about even hypothesized effects of D2 desensitization as it seems important from this, or something on this page about peptide action
December 15, 2019 at 11:25 am
So the point of the Kappa and Mu receptor section as a whole is to discuss the basis of the major side effects to lead into the Peptide Analgesics section where we discuss why peptides can avoid these side effects as well as recent research. I have included more information about peptides in a new overview section as well as a little more on D2 autoreceptors including their general function in transmission as well as the fact the desensitization also leads to increased function as shown in the figure 15.
November 10, 2019 at 6:19 pm
What does it mean when the genes are up and down regulated in MAPK signaling? Again make a separate paragraph for the summary/conclusions to organize your info. The receptor agonism diagram is very simple and serves as a good visual.
December 15, 2019 at 10:58 pm
I added a new overview section but felt that the since there was less information than on the Mu receptor page I only needed a quick summary. Furthermore the diagrams and headers already do a good job at emphasizing the key takeaways. Instead I focused on providing a good transition to peptide therapeutics since providing a background for why peptide therapeutics are needed in the main purpose of the pathways and receptors section as previous comments suggested.
November 10, 2019 at 8:28 pm
The whole pathway of dysphoria seems a little confusing to me. I understand that the pathway is not well known but to me it seems that you are contradicting yourself when you talk about the other pathway rather than making sure to mention that it is another potential pathway. I do enjoy the way the section is written and how the message does not deviate from the mechanism. As for dysphoria you mention that it is associated with severe mental disorders. I was wondering if you could go more into that because I think I am still not understanding why if mu receptor agonists are so bad due to causing respiratory failure that kappa receptor agonists aren’t used instead in pain treatment.
December 15, 2019 at 11:02 am
I made the section a little more clear in that these are two potential pathways for the dysphoric effects but neither has been proven.
Also in terms of why kappa agonists aren’t already used mu agonists were also synthesized first and remain better characterized than kappa agonist. Additionally mu agonists do not always cause addiction as shown by the continued use of morphine in all major hospitals and it’s lethal effects only happen after ingesting a very high quantity. Finally prescribing a drug with intense dysphoric side effects to someone already in a great deal of pain is not ideal. As a result, especially in the case of drug development which takes a long time, kappa agonists were not a main priority in the past.