Vision & Goals
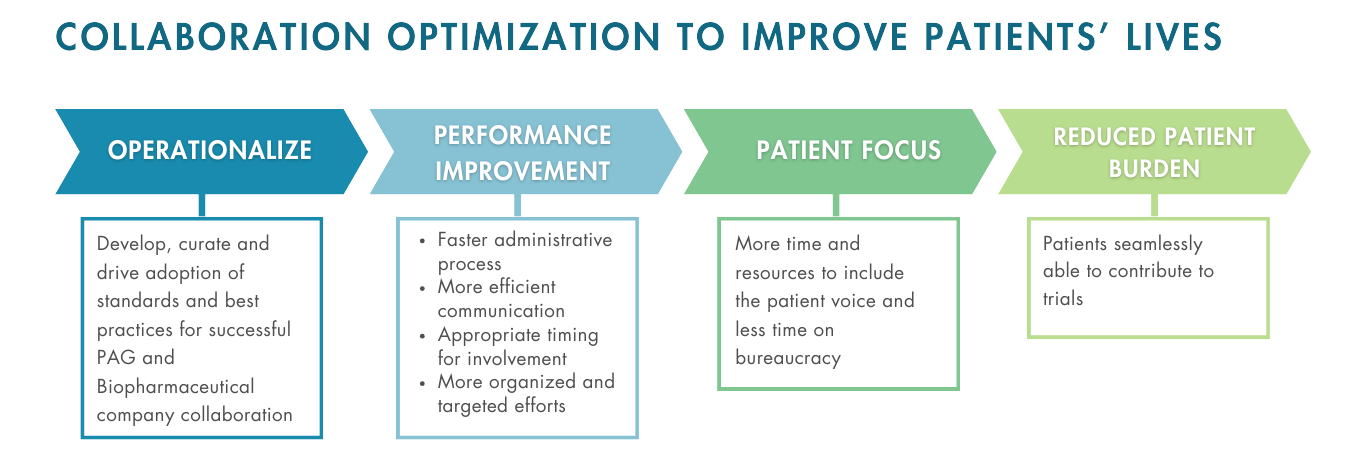
PALADIN is a pre-competitive, disease-agnostic joint consortium of 20+ patient advocacy groups (PAGs) and biopharmaceutical companies (Industry) aimed to build consensus driven guidelines & published resources to transform how PAGs and Industry collaborate to speed medicines development.
In February 2023, over 20 charter groups held a kick-off meeting to collectively develop:
- common foundations that improve Industry and PAG R&D-focused collaborations;
- guidance and trainings for PAG and Industry representatives on how to implement patient and caregiver informed R&D approaches;
- measures to improve diversity in clinical trials through best-practice sharing;
- guidance and resources to improve clinical trial participation; and
- increased knowledge-sharing about effective solutions to transform the pace of medicines development.
PALADIN Goals in 2024:
- Evaluate the Utility and Value of PALADIN’s Launch Year Deliverables
- Continue Skills, Training and Framework Development for Effective Collaboration
- Establish consensus-based metrics to evaluate the overall performance and success of how PAGs and Industry Collaborate
Looking for a quick reference that describes PALADIN or want to share what we’re about with colleagues at your organization or beyond? Please fill out the form below to view or download the PALADIN informational one-pager.