The Answer
When you eat, insulin is secreted to restore blood-glucose homeostasis and to tell your body to store energy. When you overeat, the excess nutrients are stored as glycogen for future energy use (5). Eventually, repeated overeating has consequences, namely, insulin resistance and metabolic stress. These consequences will cause the Inflammatory Signaling Pathway to activate (6).
In the Inflammatory Signaling Pathway, immune cells are recruited to help combat metabolic stress by inducing cytokine production. Cytokines are pro-inflammatory molecules that promote cellular inflammation. The build-up of immune cells, and subsequently the build-up of cytokines, encourages inflammation (8). When the body is constantly having to recruit more immune cells to fight the metabolic stress caused by overeating, the result will be a chronic state of low-grade inflammation.
The Inflammatory Signaling Pathway is activated by transcription of NF-kB protein, which activates inflammatory cytokines from immune cells. Our bodies have visceral adipose tissue (VAT), and when the NF-kB signals the inflammatory pathways, localized inflammation develops in the VAT (9). This “cause[s] recruitment of further immune cells, thereby inducing a vicious cycle of chronic low-grade inflammation” (10). Inhibiting the NF-kB pathway reduces the production of pro-inflammatory cytokines, therefore reducing inflammation in the cells (9).
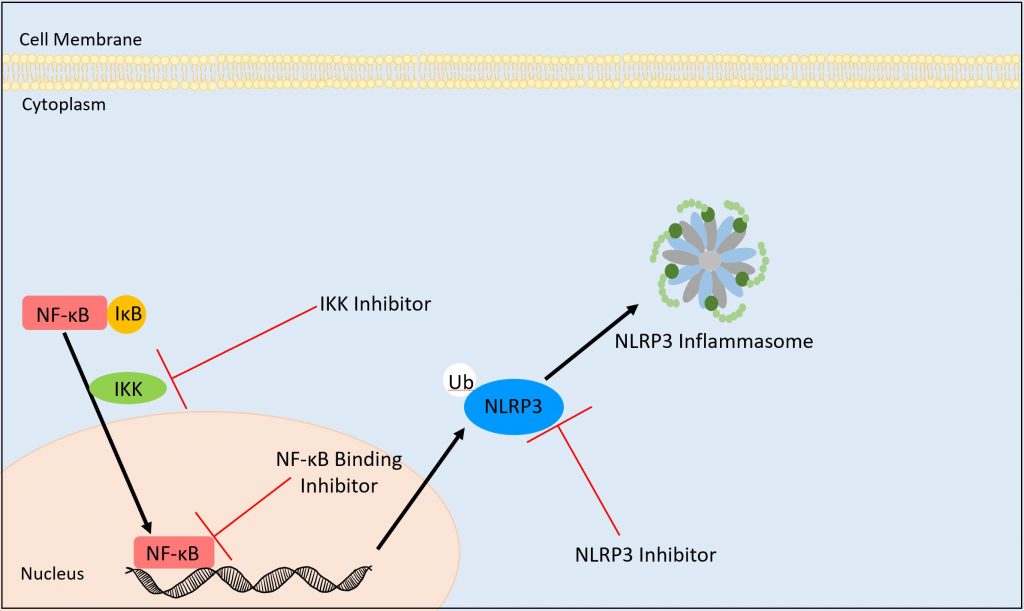
Image adapted from NF-kB Signaling in Inflammation (9)
In the Inflammatory Signaling pathway, there are multiple points of potential inhibition to combat inflammation. In order for NF-kB to enter into the nucleus, I-kappa-B kinase (IKK) complex must bind to it. If this binding is inhibited, then NF-kB will not be able to enter into the nucleus and DNA transcription will not occur.
If NF-kB enters the nucleus, the next potential point of inhibition would be when NF-kB binds to the DNA for transcription. Because this DNA transcription is gene-dependent, if this binding event does not occur, then the DNA will not be transcribed and the NLRP3 agonist will not be produced.
If transcription of the gene-dependent DNA occurs and the NLRP3 agonist is produced, an NLRP3 inhibitor is the last option to inhibit binding. The NLRP3 inhibitor can prevent binding of the NLRP3 agonist to the NLRP3 inflammasome, thereby decreasing inflammation in the cell.
If any of these inhibition events occur, then the NLRP3 inflammasome will not be activated within the cell. By inhibiting inflammasome activity, the state of chronic low-grade inflammation could be reduced. Of course, this inhibition would have to happen in multiple cells in order for their to be an observable impact (9).
Recent Comments