Paper 1
Diet-induced obesity increases NF-κB signaling in reporter mice
By Harald Carlsen, Fred Haugen, Susanne Zadelaar, Robert Kleemann, Teake Kooistra, Christian A. Drevon, Rune Blomhoff
Introduction
The nuclear factor (NF)-κB is a critical pathway in the inflammatory response. It is suspected that this pathway is associated to obesity. Over the course of 12 weeks, luciferase reporter mice were put on a high-fat diet (HFD) or low-fat diet (LFD). In vivo imaging of luciferase activity showed that the NF-κB activity was higher in HFD mice compared with LFD mice.
Background
Obesity, or increased adipose tissue mass, is associated with reduced insulin sensitivity. Reduced insulin sensitivity is a major risk factor for the development of insulin resistance, a feature of type-2 diabetes and metabolic syndrome.
NF-κB can be activated in most cell types and is part of the response to foreign pathogens and stress. Its activation has also been found to be important for development of insulin resistance. This study aims to discover when, in the process of weight gain, NF-κB signaling increases. Further, the study aims to identify the time course of inflammation, organs involved, and dynamics of inflammation in relation to obesity on an HFD.
To study this, non-invasive in vivo molecular imaging of luciferase activity was captured. The luciferase activity directly reflects NF-κB transactivation because the luciferase gene is specifically controlled by 3 NF-κB DNA-binding sites. It should be noted that this is not normally the role of NF-κB and that this is a human-created situation.
Methods
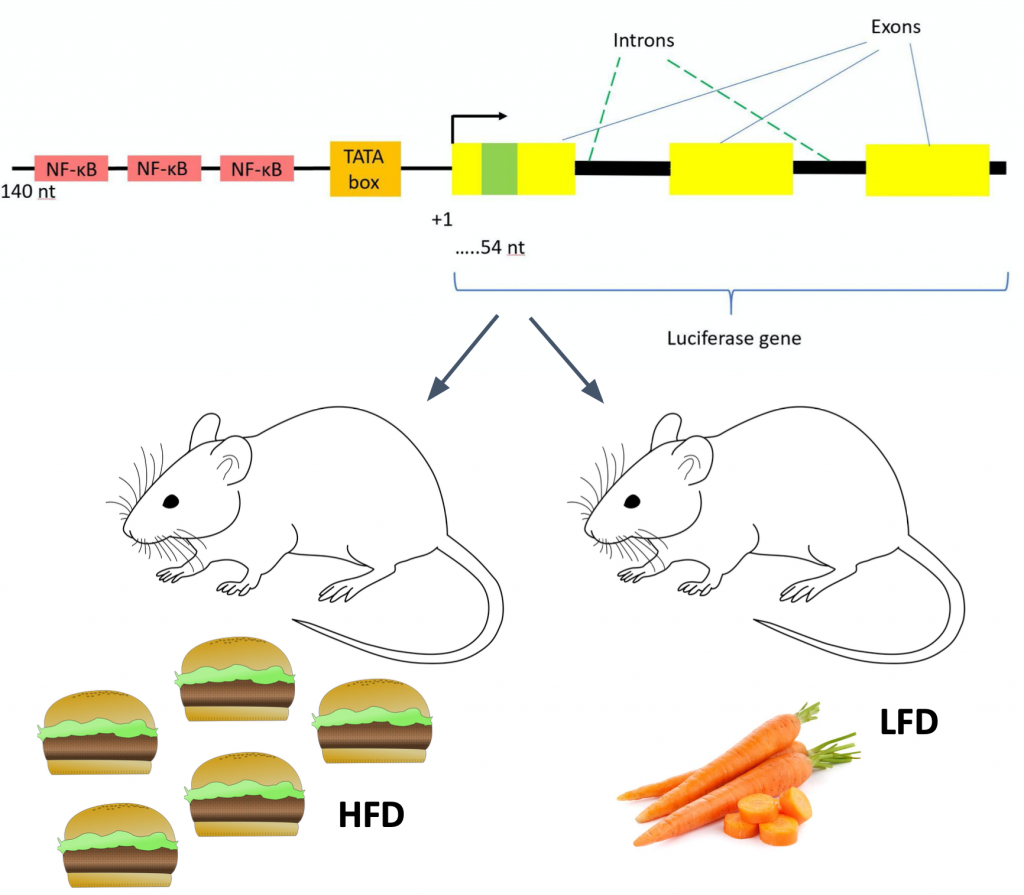
Source: Luciferase gene diagram is from Figure 22 on the Methods Page. Other images are licensed under the Creative Commons attribution-share alike 4.0 international license and were found on here.
Results
Whole Body NF-κB Activity
After 12 weeks, NF-κB activity in HFD mice increased 3.5-fold compared with baseline values while LFD increased 2.3-fold. Upon analysis of various body regions (thoracic and abdominal) and sex sub-groups, it was found that NF-κB activity differs depending on these specifications. The thoracic region of HFD females experienced the largest increase in NF-κB compared to LFD females; male HFD mice experienced decreased NF-κB activity compared to LFD males. The abdominal region of HFD males experienced an increase in NF-κB compared to LFD males; the same was not true for females.
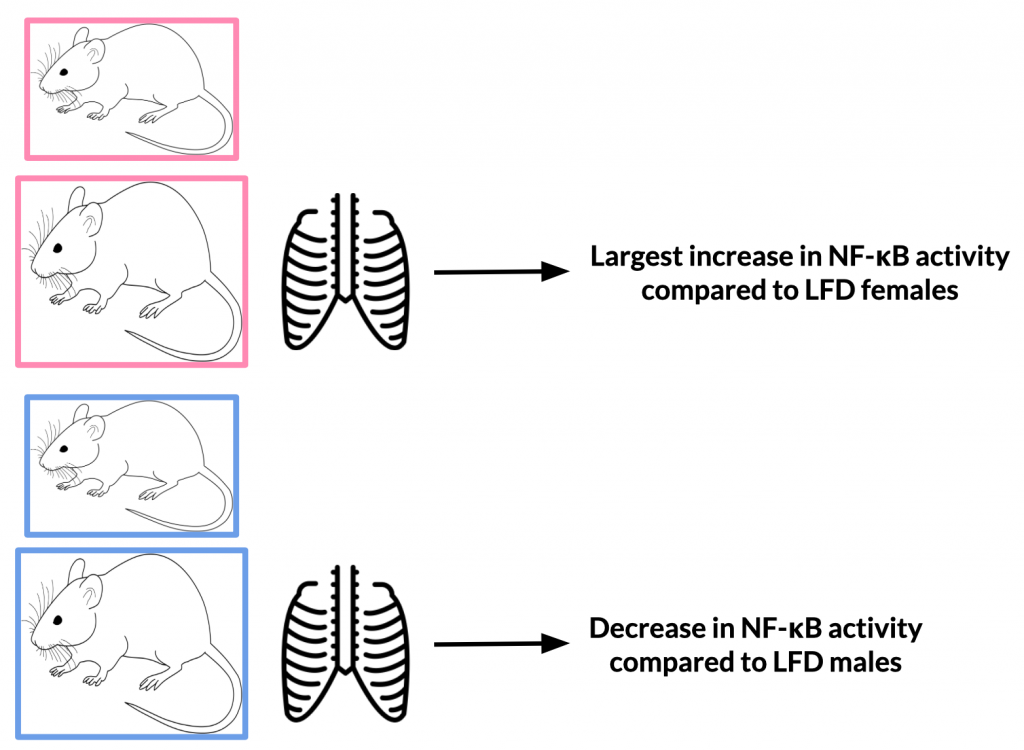
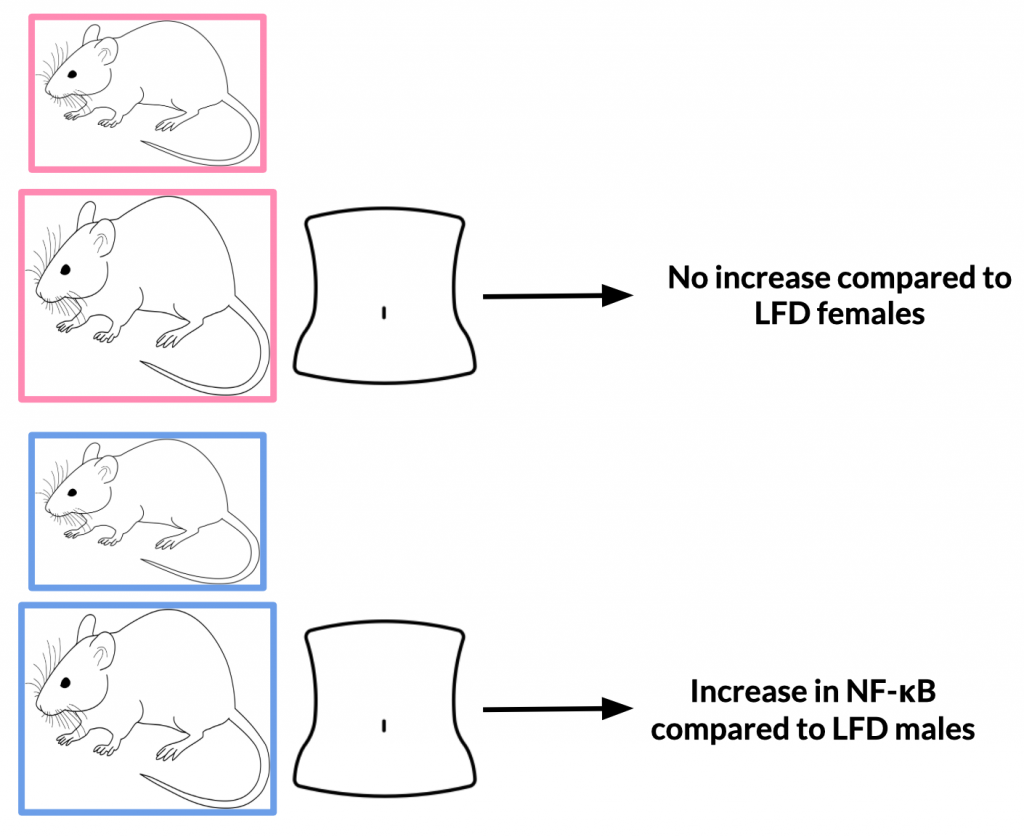
Variance in results from males to females may be due to differences in hormone levels. For example, it is possible that enhanced NF-κB activity in HFD females could be explained by elevated leptin levels. This could be applicable to studies on humans when male and females also have different levels of the same hormone.
Plasma markers of inflammation showed that the local inflammatory NF-κB response translated into a systemic inflammatory response when mean plasma IL-6 concentrations in HFD mice were higher than in LFD mice.
Thoracic NF-κB Activity and Relative Body Weight Gain
A positive correlation was found between relative increase in body weight from baseline and thoracic NF-κB activity in HFD. Enhanced body weight also yielded two to threefold increased plasma leptin levels in HFD compared with LFD.
Abdominal NF-κB Activity and Glucose Intolerance
HFD males developed glucose intolerance by week 6 of feeding and was also observed after 11 weeks of feeding. Females never developed glucose intolerances. It was also found that individual NF-κB activity does not correlate with the individual degree of glucose intolerance achieved by HFD feeding.
Discussion
Mice-fed HFD displayed 3.5-fold increased whole body NF-κB activity which shows that basal NF-κB activity increases in a time-dependent manner. NF-κB activity increased with HFDs but in a sex-dependent manner and was localized to different body regions. Females on the HFD experienced increased NF-κB activity in the thymus and/or thoracic lymph nodes. As mentioned earlier, it is possible that this is due to elevated leptin levels. In fact, it is also known that human women tend to have higher leptin levels than men, which is independent of the extent to which a person is obese (29). Kennedy et al. presume that these difference in leptin levels are due to gender-based differences in regulation and action of leptin in humans (29).
The abdominal region was not universally the major site of HFD-induced NF-κB activity associated with glucose intolerance. However, the HFD males did experience a modest increase in abdominal NF-κB activity. No intraindividual correlation was found between abdominal NF-κB and glucose intolerance.
Based off of visual inspection, it is likely that NF-κB activity arises in visceral fat and/or mesenteric lymph nodes, and not from liver.
While the typical HFD and obesity activation of NF-κB is about twofold, the typical activation of acute inflammatory reactions is 10-100-fold. This goes to show that obesity is a chronic low-grade inflammatory condition.
Summary
This paper assessed NF-κB activity in HFD and LFD mice. NF-κB activity is elevated in mice-fed HFD. Further, weight gain may be a predictor of NF-κB activity in the thoracic region of female mice.
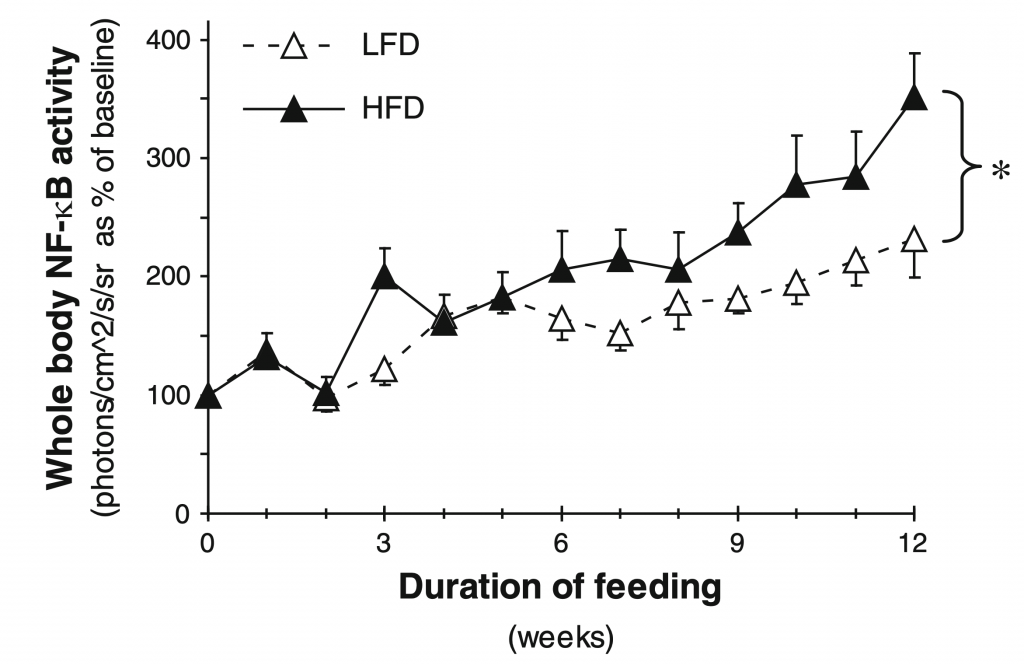
Figure taken from Carlsen et al. (26) (Figure 2)
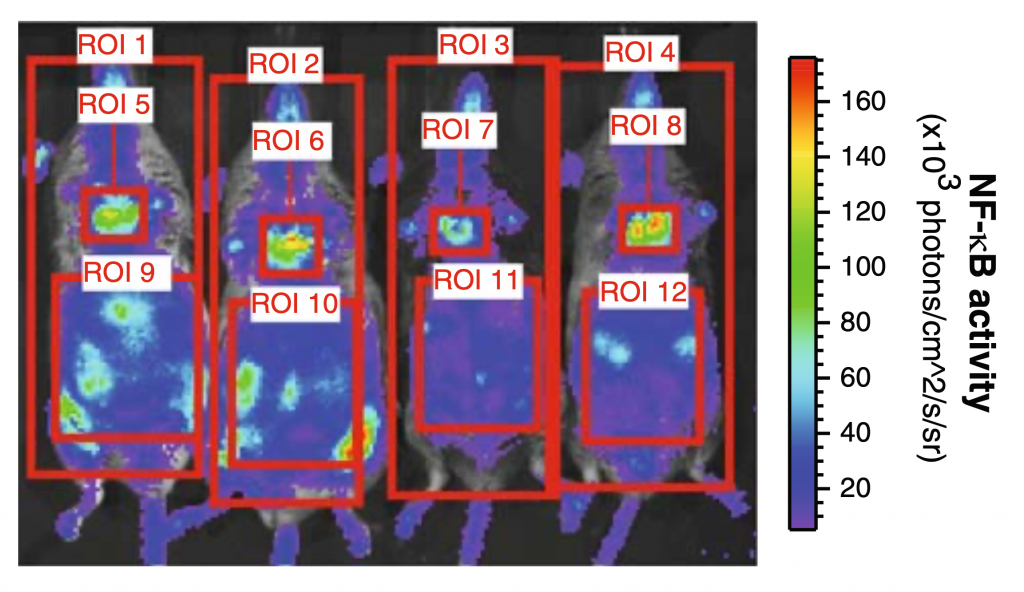
Figure taken from Carlsen et al. (26) (Figure 1)
Recent Comments