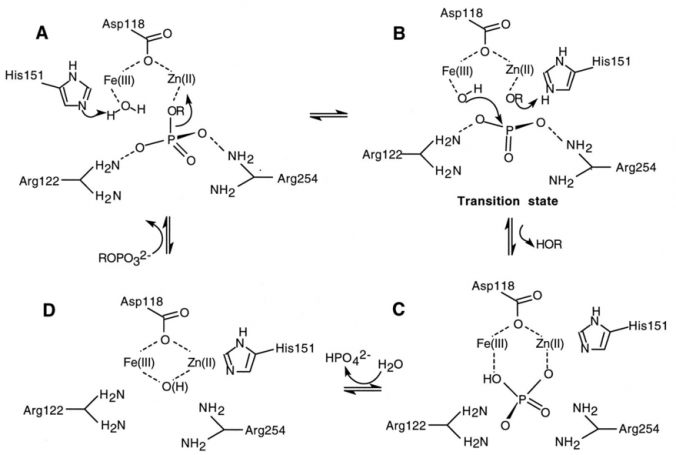
A proposed mechanism for the serine threonine phosphatase activity of calcineurin is shown. Calcinuein activates a water molecule while using its cofactor zinc to remove NFAT from one of its phosphate groups. The activated hydroxyl then attacks the phosphate group and NFAT is protonated. NFAT leaves the active site and the phosphate group is held in a coordinated complex with Iron and Zinc. Upon the addition of water the phosphate leaves the active site and Cn is ready to dephosphorylate another NFAT molecule (17).
Leave a Reply