Mechanism for Hepatic Necrosis
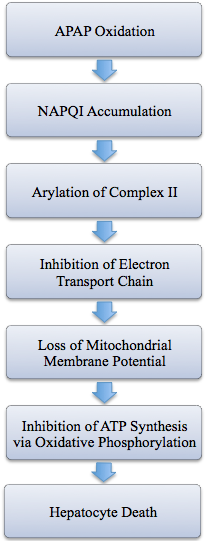
NAPQI ultimately causes liver failure by disrupting the mitochondrial membrane potential in liver cells, thus preventing ATP synthesis by oxidative phosphorylation. The exact mechanism for how NAPQI causes cell death is complex and incompletely understood. However, the molecule is highly reactive and has been found to covalently bond with several proteins due to its electrophilicity and ability to irreversibly arylate thiol residues (19). In one study, researchers radioactively labelled APAP and administered a lethal dose to mice. Two hours post drug administration, the mice were killed for their tissues to be analyzed. Researchers found high levels of radioactivity in liver samples compared to other tissue, and more importantly, they noticed that the amount of APAP administered directly corresponded with the severity of liver necrosis and the amount of radioactivity found in liver cells (20). This provides evidence that irreversible binding of NAPQI to liver cell proteins causes hepatic necrosis.
Other studies have concluded that NAPQI concentration correlates with electron transport chain (ETC) activity. In particular, it is hypothesized that NAPQI inhibits succinate dehydrogenase (complex II) activity. In one experiment, researchers found that a10µL concentration of NAPQI decreased succinate dehydrogenase activity by 45%, while a 50µL concentration completely inhibited enzymatic activity. By comparison, the substance had little to no effect on the functionality of other ETC enzymes and ATP synthase. The structure of succinate dehydrogenase might provide a molecular explanation for its inhibition by NAPQI; it contains several cysteine-rich sulfur clusters whose sulfhydryl groups are potential sites for arylation by NAPQI (21). Overall, several studies indicate that high levels of APAP cause a loss of mitochondrial membrane potential, a decrease in ATP to ADP ratio in the cytoplasm, and the irreversible covalent attachment of NAPQI to liver proteins, but more research is needed to gain a full understanding of the effects of NAPQI on liver cells.
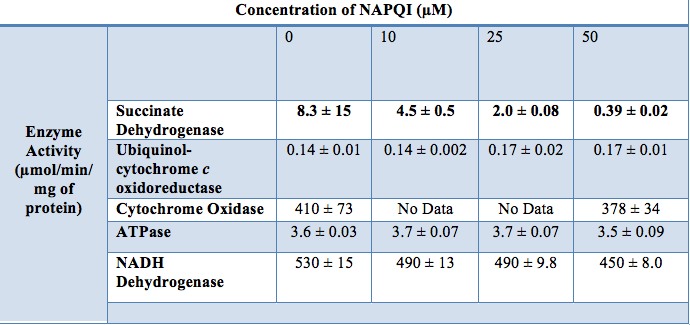
Figure 21: Complex II activity decreased as [NAPQI] increased. Other ETC enzymes were mostly unaffected by different [NAPQI] (21).
November 10, 2016 at 6:01 pm
This is just me being annoying, but “accumulation” is spelled incorrectly in Figure 22 (sorry, I’ve remade enough figures to understand how annoying me saying this is)
In the first paragraph when you say that NAPQI binds irreversibly to liver cell proteins, are these proteins succinate dehydrogenase Complex II or other things? Your figure leads me to believe that NAPQI leads to hepatocytic death solely through ATP inhibition, but your first paragraph seems to imply it’s a two-pronged approach of ATP inhibition as well as other irreversible binding processes.
November 11, 2016 at 12:45 am
I know you said the mechanism for necrosis is not known/understood, but you said NAPQI “has been found to covalently bond with several proteins due to its electrophilicity and ability to irreversibly arylate thiol residues.” Could you show this an arrow pushing mechanism to how this occurs?
August 1, 2023 at 5:33 am
ybnpbcjo http://para-mayores.es/# lieraxpc
August 6, 2023 at 6:25 am
accutane 20 mg https://isotretinoinacne.shop/# accutane south africa
August 19, 2023 at 11:37 am
cialis dapoxetine europe tadalafilise.cyou/#
August 22, 2023 at 3:49 am
Hey! I’m at work browsing your blog from my new iphone 4! Just wanted to say I love reading your blog and look forward to all your posts! Carry on the fantastic work!
August 22, 2023 at 9:50 am
como comprar cialis cialis 20 mg daily female cialis review
August 22, 2023 at 10:32 am
I loved as much as you’ll receive carried out right here. The sketch is attractive, your authored material stylish. nonetheless, you command get bought an impatience over that you wish be delivering the following. unwell unquestionably come more formerly again since exactly the same nearly very often inside case you shield this hike.
August 24, 2023 at 11:10 am
when not to take cialis is there a generic cialis atlantic pharmacy cialis
August 25, 2023 at 7:45 am
Its like you learn my thoughts! You appear to understand a lot about this, like you wrote the ebook in it or something. I think that you could do with some percent to pressure the message house a little bit, but instead of that, this is great blog. An excellent read. I will definitely be back.
August 25, 2023 at 9:49 am
I would like to thank you for the efforts you’ve put in writing this site. I am hoping the same high-grade website post from you in the upcoming as well. In fact your creative writing abilities has inspired me to get my own web site now. Actually the blogging is spreading its wings quickly. Your write up is a great example of it.
August 25, 2023 at 5:33 pm
I believe that avoiding highly processed foods may be the first step in order to lose weight. They will often taste great, but refined foods contain very little nutritional value, making you consume more only to have enough strength to get with the day. Should you be constantly ingesting these foods, transferring to cereals and other complex carbohydrates will help you to have more vigor while consuming less. Interesting blog post.
August 25, 2023 at 6:34 pm
Wow, amazing blog layout! How lengthy have you ever been blogging for? you make running a blog glance easy. The full glance of your website is magnificent, let alone the content material!
August 26, 2023 at 9:14 am
cialis 10mg vs 20mg cialis coupons coupons for cialis
August 27, 2023 at 3:08 am
you’re really a good webmaster. The web site loading speed is amazing. It seems that you’re doing any unique trick. Also, The contents are masterwork. you have done a excellent job on this topic!
August 27, 2023 at 4:20 pm
I have really noticed that fixing credit activity really needs to be conducted with tactics. If not, you are going to find yourself endangering your position. In order to succeed in fixing your credit score you have to make sure that from this minute you pay any monthly fees promptly prior to their booked date. It is really significant given that by not necessarily accomplishing that area, all other actions that you will decide on to improve your credit positioning will not be powerful. Thanks for discussing your concepts.
August 28, 2023 at 4:41 am
10mg cialis for daily use generic cialis online canada cialis experience forum
August 28, 2023 at 6:44 pm
I would like to thnkx for the efforts you have put in writing this website. I am hoping the same high-grade website post from you in the upcoming also. Actually your creative writing abilities has encouraged me to get my own website now. Actually the blogging is spreading its wings quickly. Your write up is a great example of it.
August 30, 2023 at 11:17 am
Howdy very nice web site!! Guy .. Beautiful .. Superb .. I will bookmark your blog and take the feeds also?I am happy to seek out numerous useful information right here within the submit, we’d like work out more strategies in this regard, thanks for sharing. . . . . .
August 30, 2023 at 1:04 pm
exploring danube delta
September 1, 2023 at 6:37 am
Thanks for your posting. What I want to say is that when evaluating a good on the internet electronics store, look for a website with entire information on critical factors such as the personal privacy statement, safety details, payment methods, and various terms in addition to policies. Continually take time to read the help and also FAQ sections to get a greater idea of the way the shop operates, what they can perform for you, and in what way you can make best use of the features.
September 3, 2023 at 5:21 am
I do agree with all the ideas you have introduced to your post. They’re very convincing and will definitely work. Still, the posts are too short for beginners. Could you please extend them a little from next time? Thanks for the post.
September 4, 2023 at 3:38 am
hello there and thanks on your information ? I?ve definitely picked up something new from right here. I did then again experience some technical issues the use of this website, since I experienced to reload the website a lot of occasions previous to I could get it to load correctly. I have been pondering in case your hosting is OK? Now not that I’m complaining, but sluggish loading circumstances occasions will sometimes have an effect on your placement in google and could injury your quality ranking if advertising and ***********|advertising|advertising|advertising and *********** with Adwords. Well I am adding this RSS to my e-mail and could look out for much extra of your respective interesting content. Ensure that you replace this once more soon..
September 4, 2023 at 1:38 pm
great put up, very informative. I wonder why the other experts of this sector don’t understand this. You should continue your writing. I am confident, you have a great readers’ base already!
September 4, 2023 at 9:31 pm
Thanks for the something totally new you have uncovered in your post. One thing I want to touch upon is that FSBO relationships are built over time. By introducing yourself to the owners the first saturday their FSBO is usually announced, ahead of the masses begin calling on Monday, you develop a good interconnection. By sending them tools, educational supplies, free records, and forms, you become a good ally. By subtracting a personal desire for them in addition to their situation, you generate a solid connection that, on many occasions, pays off in the event the owners opt with an adviser they know plus trust — preferably you.
September 7, 2023 at 2:16 am
I additionally believe that mesothelioma is a extraordinary form of many forms of cancer that is generally found in these previously exposed to asbestos. Cancerous tissue form inside the mesothelium, which is a shielding lining that covers the majority of the body’s bodily organs. These cells generally form inside the lining of your lungs, tummy, or the sac which actually encircles the heart. Thanks for expressing your ideas.
September 7, 2023 at 4:39 am
I’m in awe of the author’s talent to make complicated concepts accessible to readers of all backgrounds. This article is a testament to his expertise and commitment to providing valuable insights. Thank you, author, for creating such an compelling and enlightening piece. It has been an absolute pleasure to read!