Methods
A study conducted by Nabeeth Mourad and Charles L. Woronick reported the discovery of the first crystal structure of alcohol dehydrogenase from human liver through a purification procedure that used fractionation with ammonium sulfate, treatment with ethanol-chloroform, chromatography on carboxymethylcellulose and on diethylaminoethylcellulose, and crystallization from aqueous ethanol. Utilizing ultracentrifugation and polyacrylamide gel disc electrophoresis, the crystallized protein was determined to be homogenous.Using the results from horse liver ADH in which the rate constant for alcohol oxidation at 37ºC is 1.4 times as great as the rate constant at 23ºC, they calculated the rate for human liver ADH at 37ºC to be 55% as great as the actual rate at which ethanol is oxidized. This is evidence of the fact that not all the ethanol in the human body is oxidized by liver ADH, as there are ADH isoforms in the kidney and erythrocytes, and some of the ethanol isn’t oxidized at all but directly excreted (22).
A study carried out by Wang et al. used Gaussian 03, a computational program, to complete density-functional calculations for the three proposed mechanisms for the oxidation of ethanol by CYP2E1. The active site was modeled using an oxoferryl species Fe4+O2-(C20N4H12)–(SH)– with ethanol used as the substrate. The method was chosen to be UB3LYP functional, which is spin-unrestricted, and Wang et al. coupled it with LACVP** in which all geometries were optimized at the UB3LYP/LACVP** level. They determined that transition states contained one imaginary frequency mode through vibrational analysis. Chlorobenzene was used as the solvent in single-point energy and self-consistent reaction field (SCRF) calculations in order to determine the effects of the weak polarization environment of the heme pocket using the polarizable continuum model solvation method. SCRF calculations were also carried out in a polar environment to model the polarity effects of high concentrations of ethanol in the heme pocket. The results of these calculations led the researchers to determine that the active site of CYP2E1 is composed of almost degenerate low-spin doublet and high-spin quartet states. After determining this spin-state duality in the active site, they studied the three mechanisms on both spin-state surfaces, leading them to the conclusion that ethanol oxidation occurs through the reverse dual-hydrogen abstraction (R-DHA) mechanism in polar environments while in nonpolar environments the R-DHA mechanism competes with the gem-diol mechanism to oxidize ethanol (8).
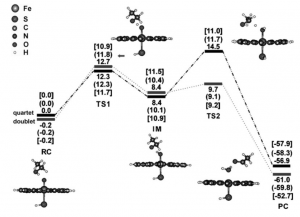
Figure 22: The UB3LYP/LACVP** energy profile determined by SCRF calculations for ethanol oxidation in the active site of CYP2E1 through the R-DHA mechanism. The energies shown depict the gas phase (no parentheses), the weak-polar medium (parentheses), and the polar medium (brackets). RC refers to the reactants complex, IM to the intermediate complex, PC to the acetaldehyde complex, and TS to the transition states (8).
To learn more about the results of this method, click here
November 10, 2016 at 6:34 pm
Great page! Some of this definitely went over my head but I liked the references to MO theory. Since this page is pretty dense, would it be possible to give definitions of some of the more specific terms? In particular, I had to look up UB3LYP, LACVP** , Gaussian 03, and R-DHA (which I now realize you defined in an earlier page but had forgotten by the time I made it to this one)
August 2, 2023 at 2:40 pm
feqoclka http://para-mayores.es/# aufmrnvj
August 20, 2023 at 5:12 pm
20mg cialis daily cialtadamdv.com tadalafilise.cyou/#
August 22, 2023 at 4:05 am
Howdy! I know this is kinda off topic but I was wondering if you knew where I could find a captcha plugin for my comment form? I’m using the same blog platform as yours and I’m having problems finding one? Thanks a lot!
August 23, 2023 at 1:30 pm
cialis blue pills otc cialis 2017 generic for cialis tadalafil
August 23, 2023 at 3:33 pm
Thanks for your concepts. One thing really noticed is always that banks plus financial institutions know the spending patterns of consumers and as well understand that a lot of people max outside their real credit cards around the breaks. They wisely take advantage of this kind of fact and commence flooding your own inbox in addition to snail-mail box having hundreds of no-interest APR card offers soon after the holiday season concludes. Knowing that for anyone who is like 98 of the American open public, you’ll hop at the one opportunity to consolidate credit debt and transfer balances towards 0 annual percentage rates credit cards.
August 24, 2023 at 9:32 pm
You actually make it seem so easy with your presentation but I find this matter to be really something that I think I would never understand. It seems too complex and extremely broad for me. I’m looking forward for your next post, I?ll try to get the hang of it!
August 25, 2023 at 2:46 pm
does cialis help women cialis forum viagra vs cialis
August 26, 2023 at 11:19 am
It’s my belief that mesothelioma is most deadly cancer. It contains unusual traits. The more I look at it the greater I am confident it does not react like a true solid human cancer. In the event mesothelioma is often a rogue virus-like infection, therefore there is the probability of developing a vaccine along with offering vaccination for asbestos open people who are open to high risk regarding developing long term asbestos associated malignancies. Thanks for discussing your ideas on this important health issue.
August 26, 2023 at 6:02 pm
I have been browsing online greater than 3 hours these days, but I never discovered any fascinating article like yours. It?s beautiful value sufficient for me. In my view, if all site owners and bloggers made good content as you probably did, the net might be a lot more helpful than ever before.
August 27, 2023 at 6:08 am
Hi there! Someone in my Myspace group shared this site with us so I came to check it out. I’m definitely enjoying the information. I’m book-marking and will be tweeting this to my followers! Exceptional blog and outstanding style and design.
August 27, 2023 at 9:17 am
tadalafil vendita online tadalafil price original cialis 20mg
August 29, 2023 at 3:52 am
I do not even know how I ended up here, but I thought this post was good. I do not know who you are but certainly you’re going to a famous blogger if you aren’t already 😉 Cheers!
August 31, 2023 at 2:18 am
Wonderful blog! I found it while surfing around on Yahoo News. Do you have any tips on how to get listed in Yahoo News? I’ve been trying for a while but I never seem to get there! Thank you
August 31, 2023 at 8:59 am
This is hands down one of the greatest articles I’ve read on this topic! The author’s comprehensive knowledge and enthusiasm for the subject shine through in every paragraph. I’m so thankful for stumbling upon this piece as it has enriched my knowledge and sparked my curiosity even further. Thank you, author, for investing the time to produce such a remarkable article!
September 1, 2023 at 10:16 pm
Great write-up, I?m normal visitor of one?s web site, maintain up the nice operate, and It’s going to be a regular visitor for a long time.
September 2, 2023 at 5:57 pm
We’re a group of volunteers and opening a new scheme in our community. Your web site provided us with helpful information to paintings on. You have done an impressive process and our entire group will be thankful to you.
September 3, 2023 at 10:16 am
I am often to blogging and i really admire your content. The article has actually peaks my interest. I am going to bookmark your site and maintain checking for new information.
September 4, 2023 at 2:59 am
Howdy! Do you know if they make any plugins to safeguard against hackers? I’m kinda paranoid about losing everything I’ve worked hard on. Any tips?
September 5, 2023 at 7:21 pm
Thanks for your thoughts. One thing I have noticed is that often banks and also financial institutions are aware of the spending behaviors of consumers as well as understand that most of the people max outside their credit cards around the vacations. They wisely take advantage of this particular fact and commence flooding ones inbox along with snail-mail box along with hundreds of 0 APR credit cards offers immediately after the holiday season comes to an end. Knowing that in case you are like 98 in the American community, you’ll soar at the one opportunity to consolidate credit card debt and shift balances towards 0 rate credit cards.