Toxicity
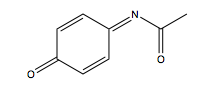
APAP hepatotoxicity does not originate from the molecule itself but is rather attributed to one of its metabolites, N-acetyl-p-benzoquinone imine (NAPQI). This metabolic intermediate forms upon oxidation of APAP via CYP2E1 enzyme. Normally, NAPQI is quickly conjugated with glutathione (GSH) via glutathione transferase to produce the non-toxic compound APAP-GSH. However, a rapid influx of NAPQI depletes GSH stores, causing NAPQI to build up in liver cells (15). For this reason, patients who overdose on APAP are treated with N-Acetyl Cysteine (NAC), which stimulates GSH production to protect against liver damage (16).
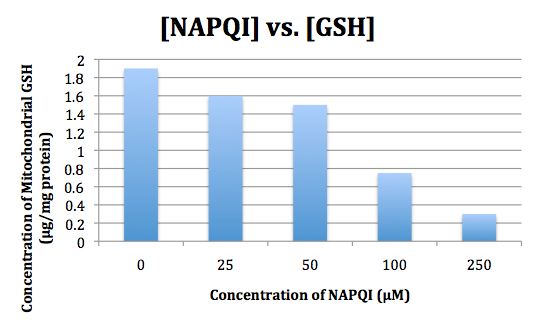
Figure 18: NAPQI depletes GSH stores (21).
To see a full scheme of the APAP metabolism and to learn about its interaction with ethanol, click here.
November 7, 2016 at 7:56 pm
I love the bar graph, since I think it does a really good job of showing the relationship between NAPQI and GSH.
I think it could be beneficial to show a figure which shows the pathways you describe on this slide. Can you show the conjugation of NAPQI and GSH to produce APAP-GSH? Also I know yo explain N-acetyl cysteine later, so maybe include a hyperlink to that page? When I first read it, I was left wanting to know more about this antidotal process.
November 10, 2016 at 5:48 pm
Delia, I think the pathways are described/shown in more detail on their “interactions with ethanol” subpage.
Maybe when you include the hyperlink to the N-acetyl cysteine page you can also include a hyperlink to the interactions with ethanol page.
August 22, 2023 at 3:43 am
Its like you read my mind! You appear to know a lot about this, like you wrote the book in it or something. I think that you could do with a few pics to drive the message home a little bit, but instead of that, this is wonderful blog. An excellent read. I will definitely be back.
August 23, 2023 at 3:38 pm
Terrific work! This is the type of info that should be shared around the web. Shame on the search engines for not positioning this post higher! Come on over and visit my web site . Thanks =)
August 24, 2023 at 7:35 pm
Generally I do not learn post on blogs, but I wish to say that this write-up very pressured me to check out and do so! Your writing taste has been amazed me. Thank you, quite great post.
August 25, 2023 at 7:25 am
I discovered your weblog web site on google and test a number of of your early posts. Continue to keep up the superb operate. I simply additional up your RSS feed to my MSN Information Reader. Searching for ahead to reading extra from you in a while!?
August 26, 2023 at 3:03 am
Howdy! This is my first visit to your blog! We are a collection of volunteers and starting a new project in a community in the same niche. Your blog provided us valuable information to work on. You have done a marvellous job!
August 27, 2023 at 4:57 am
Just wish to say your article is as astounding. The clearness in your post is simply nice and i can assume you are an expert on this subject. Well with your permission let me to grab your feed to keep up to date with forthcoming post. Thanks a million and please carry on the gratifying work.
August 27, 2023 at 5:56 pm
I just could not depart your site before suggesting that I extremely enjoyed the standard information a person provide for your visitors? Is gonna be back often to check up on new posts
August 27, 2023 at 9:13 pm
Heya i am for the first time here. I came across this board and I find It truly useful & it helped me out much. I hope to give something back and help others like you helped me.
August 28, 2023 at 5:26 am
Magnificent website. A lot of useful information here. I?m sending it to some friends ans also sharing in delicious. And naturally, thanks for your effort!
August 29, 2023 at 11:01 am
Thanks for your article. Another item is that to be a photographer requires not only issues in taking award-winning photographs but also hardships in acquiring the best photographic camera suited to your needs and most especially challenges in maintaining the grade of your camera. It is very true and visible for those professional photographers that are in to capturing the particular nature’s captivating scenes — the mountains, the actual forests, the particular wild or seas. Going to these exciting places undoubtedly requires a photographic camera that can meet the wild’s harsh settings.
August 30, 2023 at 7:33 am
Thanks , I have just been looking for information about this subject for ages and yours is the greatest I have discovered so far. But, what about the conclusion? Are you sure about the source?
August 30, 2023 at 9:54 am
I have discovered some important matters through your site post. One other subject I would like to convey is that there are numerous games that you can buy which are designed specially for preschool age children. They include things like pattern recognition, colors, wildlife, and styles. These normally focus on familiarization rather than memorization. This keeps a child occupied without feeling like they are studying. Thanks
August 31, 2023 at 12:12 am
pescuit murighiol
August 31, 2023 at 12:36 pm
Nice post. I study something more challenging on totally different blogs everyday. It is going to at all times be stimulating to read content from other writers and apply just a little something from their store. I?d prefer to use some with the content material on my blog whether or not you don?t mind. Natually I?ll provide you with a hyperlink on your web blog. Thanks for sharing.
September 1, 2023 at 11:07 pm
Does your blog have a contact page? I’m having a tough time locating it but, I’d like to shoot you an email. I’ve got some creative ideas for your blog you might be interested in hearing. Either way, great website and I look forward to seeing it improve over time.
September 2, 2023 at 12:10 am
Have you ever thought about publishing an ebook or guest authoring on other sites? I have a blog based on the same subjects you discuss and would really like to have you share some stories/information. I know my audience would enjoy your work. If you’re even remotely interested, feel free to send me an e-mail.
September 5, 2023 at 6:44 am
My brother recommended I may like this web site. He was totally right. This post actually made my day. You can not imagine just how so much time I had spent for this info! Thanks!
December 15, 2023 at 10:21 am
Your virtuosic command of language is nothing short of awe-inspiring, as you effortlessly navigate the intricate nuances of syntax and semantics, fashioning prose that transcends the realm of mere words and ascends to the pantheon of linguistic artistry.
December 23, 2023 at 9:00 am
Your blog is a haven of positivity in a sometimes chaotic online world.