Catalase
A tertiary pathway for the oxidation of ethanol is carried out by catalase, a peroxisomal enzyme that also catalyzes the removal of hydrogen peroxide (H2O2). Although catalase has a much smaller role in alcohol oxidation than ADH or CYP2E1, it is important in cerebral function as inhibiting catalase has been found to decrease the rate of oxidation of ethanol to acetaldehyde by the brain (3).
In conditions with high hydrogen peroxide concentration, catalase works to convert two molecules of H2O2 into two molecules of water. The first H2O2 molecule enters the active site where it oxidizes the haem iron to produce an oxyferryl group with a π-cationic porphyrin radical and a molecule of water. A second molecule of hydrogen peroxide then enters the active site and is oxidized to molecular oxygen and water. In low concentrations of H2O2, however, an alternative pathway will occur due to the strong oxidant nature of the oxyferryl group formed after the first molecule of H2O2 is oxidized. The hydroxyl radicals will exit the channel to the active site and oxidize ethanol molecules to acetaldehyde molecules outside of the active site (10).
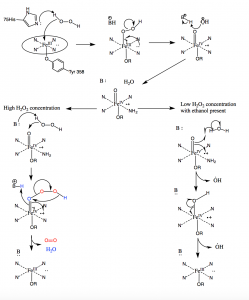
Figure 10: Mechanism of catalase. Under high hydrogen peroxide conditions, catalase follows the mechanism on the left to oxidize two peroxide molecules. Under low concentrations of hydrogen peroxide, catalase follows the radical mechanism on the right and produces two hydroxyl radicals.
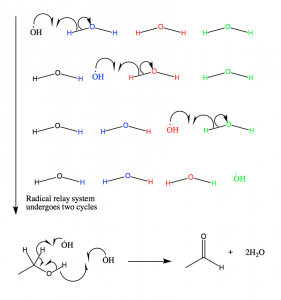
Figure 11: The hydroxyl radicals produced in the radical catalase mechanism are channeled out of the active site via this mechanism. The radicals then react with ethanol, which is held in proximity to the active site. If ethanol is not present, NADPH is oxidized.
The active site of catalase is a haem moiety with His-75 playing a major role in the catalytic activity; it uses a charge relay (residues Tyr358, Arg354, His218 and Asp348) in order to carry out reactions without disrupting peroxide binding. When the oxyferryl group is formed, the charge relay system is initiated through the continuous movement of the coordination bond electrons from one bond to the next. The resonance attributed to the movement of the coordination bond electrons allows an electron cloud to form around the iron center in which electrons can be donated to the catalytic reactions (10).
November 10, 2016 at 4:36 pm
Figure 12 looks amazing! It’s well laid out too. It might add some clarity to label to oxyferryl group within the figure, though. Also, you sort of lost me in the third paragraph when you mentioned the charge relay being in the “forward” or “reverse” directions, but other than that this is a great page.
November 10, 2016 at 5:28 pm
I think it would be beneficial to add what orange, yellow, and purple color coding represents in Figure 14.
November 11, 2016 at 1:04 am
You show the active site residues of catalase and describe the importance of the “charge relay” between the residues to carry out the reaction. Would it be possible to show a figure with these interactions of charges? And how this relates to peroxide binding?
August 1, 2023 at 6:59 am
viqtmbpd http://para-mayores.es/# enyczqlb
August 6, 2023 at 7:47 am
accutane brand name vs generic pharmacy accutane accutane buy
August 7, 2023 at 6:00 am
Hello very cool site!! Man .. Excellent .. Wonderful .. I’ll bookmark your website and take the feeds additionally?
I am satisfied to find numerous useful info here in the publish, we want work out extra techniques on this regard, thanks
for sharing. . . . . .
August 7, 2023 at 8:51 pm
Wow, this post is nice, my younger sister is analyzing such things, therefore
I am going to tell her.
August 7, 2023 at 10:19 pm
Great blog right here! Also your website so much up very fast!
What host are you the use of? Can I get your associate link on your host?
I want my website loaded up as quickly as yours lol
August 8, 2023 at 2:02 pm
Hello i am kavin, its my first time to commenting anyplace, when i
read this article i thought i could also create comment due to this sensible article.
August 8, 2023 at 4:07 pm
Do you have a spam issue on this website; I also am a blogger, and I was curious about
your situation; many of us have developed some nice practices and we are
looking to trade techniques with others, why not shoot me an email if interested.
August 8, 2023 at 6:12 pm
Why visitors still make use of to read news papers when in this technological world everything is accessible on net?
August 9, 2023 at 2:14 pm
Hello there! I could have sworn I’ve been to this
website before but after checking through some of the post I
realized it’s new to me. Anyhow, I’m definitely happy I found
it and I’ll be bookmarking and checking back frequently!
August 10, 2023 at 9:52 am
Truly no matter if someone doesn’t be aware of after that its up to other viewers that they will assist, so here it occurs.
August 10, 2023 at 11:56 am
I was wondering if you ever considered changing the structure of your website?
Its very well written; I love what youve got to say. But maybe you could a little more in the way of
content so people could connect with it better.
Youve got an awful lot of text for only having one or 2 images.
Maybe you could space it out better?
August 10, 2023 at 2:27 pm
It’s fantastic that you are getting thoughts from this
piece of writing as well as from our dialogue made here.
August 10, 2023 at 3:28 pm
I used to be able to find good info from your content.
August 10, 2023 at 9:07 pm
Thank you for the auspicious writeup. It in fact was a amusement account it.
Look advanced to more added agreeable from you!
However, how could we communicate?
August 11, 2023 at 11:45 am
Your style is very unique compared to other folks
I’ve read stuff from. I appreciate you for posting when you have the opportunity, Guess I will just book mark this
blog.
August 11, 2023 at 2:16 pm
You actually make it seem so easy with your presentation but I find
this matter to be actually something which I
think I would never understand. It seems too complex and very broad for me.
I am looking forward for your next post, I’ll try to get the hang of it!
August 12, 2023 at 2:17 am
I think this is one of the most important information for me.
And i’m glad reading your article. But want to remark on some general things, The site style is wonderful, the articles is really great :
D. Good job, cheers
August 12, 2023 at 4:26 am
I?m no longer certain where you’re getting your info, however great topic. I needs to spend some time studying more or understanding more. Thanks for fantastic info I used to be on the lookout for this info for my mission.
August 12, 2023 at 9:17 am
These are genuinely wonderful ideas in on the topic of
blogging. You have touched some good points here.
Any way keep up wrinting.
August 12, 2023 at 10:36 am
This info is invaluable. How can I find out more?
August 12, 2023 at 3:37 pm
Hi there, I enjoy reading through your post. I wanted to
write a little comment to support you.
August 12, 2023 at 5:06 pm
Valuable info. Fortunate me I discovered your web site unintentionally, and I’m shocked why this accident didn’t happened earlier!
I bookmarked it.
August 13, 2023 at 1:03 am
hello there and thank you for your info – I’ve definitely picked up something new from right here.
I did however expertise a few technical points using this
site, since I experienced to reload the website a lot of times previous to I could get it to load correctly.
I had been wondering if your web hosting is OK? Not that I am complaining, but sluggish loading instances times will very
frequently affect your placement in google and could
damage your high-quality score if ads and marketing with
Adwords. Well I’m adding this RSS to my e-mail and can look out for much more of your respective intriguing content.
Ensure that you update this again soon.
August 13, 2023 at 3:24 pm
Hello friends, its impressive piece of writing on the topic
of educationand fully explained, keep it up all the time.
August 13, 2023 at 6:31 pm
Awesome post.
August 13, 2023 at 11:39 pm
Hi my friend! I wish to say that this post is amazing, nice written and come
with approximately all significant infos. I would like to peer more posts like this .
August 14, 2023 at 1:32 am
Thanks designed for sharing such a pleasant opinion,
piece of writing is pleasant, thats why i have read it fully
August 14, 2023 at 2:31 am
This piece of writing provides clear idea for the new people of blogging, that
really how to do running a blog.
August 14, 2023 at 10:06 am
bxfs akibacom ckz wrestling
August 14, 2023 at 2:31 pm
Fine way of describing, and good paragraph to take facts regarding my presentation subject matter, which i am going to convey in institution of higher
education.
August 14, 2023 at 3:51 pm
After exploring a few of the blog posts on your site, I honestly like your
way of writing a blog. I added it to my bookmark website list and will be checking back soon. Take a look at my website as well and let
me know your opinion.
August 15, 2023 at 1:43 am
This paragraph will help the internet users for setting up new web site or even a blog from start to end.
August 16, 2023 at 4:55 pm
Hi there colleagues, how is everything, and what you would like to say concerning this piece of writing, in my
view its in fact awesome for me.
August 16, 2023 at 6:05 pm
I am curious to find out what blog platform
you are working with? I’m having some small security problems with
my latest site and I’d like to find something more secure.
Do you have any suggestions?
August 16, 2023 at 8:21 pm
Greetings from Ohio! I’m bored at work so I decided to browse your website on my iphone
during lunch break. I love the info you provide here and can’t wait
to take a look when I get home. I’m surprised at how quick your blog loaded on my cell phone ..
I’m not even using WIFI, just 3G .. Anyhow, amazing blog!
August 17, 2023 at 4:23 am
When someone writes an post he/she keeps the plan of a user in his/her brain that how a user can know it.
So that’s why this post is perfect. Thanks!
August 17, 2023 at 5:14 am
Just wish to say your article is as astounding. The clearness in your post is simply excellent and i can assume you are an expert on this subject.
Fine with your permission allow me to grab your RSS feed to keep up to
date with forthcoming post. Thanks a million and please keep up
the gratifying work.
August 17, 2023 at 8:16 pm
Useful information. Fortunate me I discovered your site unintentionally, and I’m shocked why this accident did not took
place earlier! I bookmarked it.
August 18, 2023 at 6:38 am
Great beat ! I would like to apprentice while you amend
your website, how could i subscribe for a blog site?
The account aided me a acceptable deal. I had been tiny bit acquainted of this your broadcast
provided bright clear concept
August 18, 2023 at 8:31 am
Highly descriptive article, I loved that a lot.
Will there be a part 2?
August 18, 2023 at 8:39 am
Undeniably believe that which you stated.
Your favorite justification appeared to be on the internet the easiest thing to be aware of.
I say to you, I certainly get annoyed while people think about
worries that they plainly do not know about. You managed to hit
the nail upon the top as well as defined out the whole thing without
having side effect , people could take a signal. Will likely be back to get more.
Thanks
August 18, 2023 at 12:32 pm
Hello there! This is my first visit to your blog!
We are a team of volunteers and starting a new initiative in a
community in the same niche. Your blog provided us valuable information to work on.
You have done a extraordinary job!
August 19, 2023 at 12:36 pm
Thanks for finally talking about > Catalase –
Alcohol Metabolism < Loved it!
August 19, 2023 at 12:51 pm
pharmacy direct cialis tadalafilise.cyou/#
August 19, 2023 at 2:10 pm
Its such as you read my thoughts! You seem to grasp so
much about this, such as you wrote the guide in it or something.
I feel that you simply can do with some p.c. to force the message house a bit, but instead of that, that is fantastic
blog. An excellent read. I’ll definitely be back.
August 19, 2023 at 5:59 pm
Hi, i read your blog occasionally and i own a similar one and i was
just curious if you get a lot of spam remarks? If so how do you prevent it, any plugin or anything you
can suggest? I get so much lately it’s driving me crazy so any assistance is very much appreciated.
August 20, 2023 at 12:42 am
Magnificent beat ! I wish to apprentice while you amend your web site, how could i subscribe for a blog site?
The account helped me a acceptable deal. I had been tiny bit acquainted of this your broadcast offered bright clear concept
August 20, 2023 at 3:51 am
Hi there, i read your blog occasionally and i own a similar one
and i was just curious if you get a lot of spam feedback?
If so how do you stop it, any plugin or anything you can advise?
I get so much lately it’s driving me mad so any assistance is very much appreciated.
August 20, 2023 at 5:35 am
you are actually a just right webmaster. The web site loading speed is amazing.
It seems that you’re doing any distinctive trick.
Moreover, The contents are masterpiece. you have done a excellent process in this matter!
August 20, 2023 at 3:13 pm
Definitely consider that that you stated. Your favourite reason seemed to be on the net
the easiest thing to take into account of. I say to you,
I certainly get annoyed even as people think about concerns that they just don’t know about.
You controlled to hit the nail upon the top and outlined
out the whole thing without having side effect , other
folks could take a signal. Will probably be again to get more.
Thank you
August 20, 2023 at 5:02 pm
Pretty! This has been an incredibly wonderful article.
Many thanks for supplying this info.
August 20, 2023 at 5:30 pm
Whoa! This blog looks just like my old one! It’s on a entirely different subject but it has pretty much the same page layout and design. Superb choice of colors!
August 20, 2023 at 8:34 pm
Hi there, all is going sound here and ofcourse every one
is sharing information, that’s actually good, keep up writing.
August 20, 2023 at 10:14 pm
Amazing blog! Do you have any suggestions for aspiring writers?
I’m planning to start my own website soon but I’m
a little lost on everything. Would you suggest starting with a
free platform like WordPress or go for a paid option? There are so many options out there
that I’m completely overwhelmed .. Any ideas?
Thank you!
August 22, 2023 at 12:19 am
As I site possessor I believe the content matter here is rattling wonderful , appreciate it for your efforts. You should keep it up forever! Good Luck.
August 22, 2023 at 3:04 am
Thanks for these pointers. One thing I additionally believe is that often credit cards providing a 0 apr often lure consumers along with zero rate of interest, instant acceptance and easy over-the-internet balance transfers, however beware of the number one factor that is going to void your own 0 easy road annual percentage rate and also throw you out into the terrible house rapidly.
August 22, 2023 at 3:51 am
Hello, you used to write fantastic, but the last several posts have been kinda boring? I miss your tremendous writings. Past several posts are just a bit out of track! come on!
August 22, 2023 at 11:02 am
cialis 10 mg effectiveness insuffisance cardiaque congestive tadalafil daily
August 22, 2023 at 2:43 pm
I all the time used to study paragraph in news papers but now as I am a
user of internet thus from now I am using net for articles or
reviews, thanks to web.
August 22, 2023 at 3:25 pm
Do you have any video of that? I’d want to
find out more details.
August 22, 2023 at 9:11 pm
Thank you for every other informative site. The place
else may just I get that kind of info written in such a perfect way?
I’ve a project that I’m simply now working on, and I’ve
been at the glance out for such information.
August 22, 2023 at 10:32 pm
Hello! I could have sworn I’ve been to this site before but after reading through some of the post I realized it’s new to me. Anyways, I’m definitely happy I found it and I’ll be book-marking and checking back frequently!
August 22, 2023 at 10:57 pm
If you are going for most excellent contents like me, just
pay a visit this website daily as it provides quality contents, thanks
August 23, 2023 at 1:12 am
Remarkable! Its really remarkable article, I have got much clear idea on the topic of from this article.
August 23, 2023 at 4:54 am
An interesting discussion is worth comment. I think that you must write extra on this matter, it won’t be a taboo topic however typically individuals are not sufficient to speak on such topics. To the next. Cheers
August 23, 2023 at 6:06 am
I constantly spent my half an hour to read this webpage’s articles
or reviews every day along with a cup of coffee.
August 23, 2023 at 10:57 pm
Wow, this piece of writing is good, my sister is analyzing such things, so I am going to convey her.
August 24, 2023 at 7:43 am
Hey there! Would you mind if I share your blog with my twitter
group? There’s a lot of folks that I think would really enjoy
your content. Please let me know. Many thanks
August 24, 2023 at 12:28 pm
discount genuine cialis cialis commercial song vardenafil vs cialis
August 25, 2023 at 1:56 am
Saved as a favorite, I like your blog!
August 25, 2023 at 4:47 am
Hey! This post could not be written any better! Reading this post reminds me of my good old room mate! He always kept talking about this. I will forward this page to him. Pretty sure he will have a good read. Many thanks for sharing!
August 25, 2023 at 5:40 am
I’ve been absent for some time, but now I remember why I used to love this web site. Thank you, I?ll try and check back more frequently. How frequently you update your web site?
August 25, 2023 at 5:55 am
I’ve learned new things through your website. One other thing I’d really like to say is that newer laptop or computer os’s tend to allow extra memory for use, but they additionally demand more memory space simply to work. If people’s computer could not handle additional memory plus the newest program requires that storage increase, it can be the time to buy a new Personal computer. Thanks
August 25, 2023 at 6:46 am
I don’t even understand how I stopped up right here, however I
assumed this submit was good. I do not recognize who you
might be however certainly you are going to a well-known blogger in case you are not already.
Cheers!
August 25, 2023 at 11:26 am
of course like your website but you need to check the spelling on quite a few of your posts. Many of them are rife with spelling problems and I find it very troublesome to tell the truth nevertheless I will certainly come back again.
August 25, 2023 at 11:30 am
https://www.masturbaza.com/
August 26, 2023 at 7:25 am
You’ve made some good points there. I checked on the web for more
information about the issue and found most individuals
will go along with your views on this web site.
August 26, 2023 at 10:17 am
siti pagamento alla consegna substitute for cialis 20 milligram cialis
August 26, 2023 at 10:55 am
Hi mates, its impressive piece of writing regarding cultureand fully
defined, keep it up all the time.
August 26, 2023 at 12:54 pm
I do love the manner in which you have presented this issue and it really does present me personally a lot of fodder for consideration. Nonetheless, coming from everything that I have witnessed, I simply just hope as other feedback pack on that men and women continue to be on point and not start on a tirade associated with the news du jour. Still, thank you for this excellent piece and although I can not go along with it in totality, I value the viewpoint.
August 26, 2023 at 2:55 pm
Hmm it appears like your blog ate my first comment (it was extremely long) so I guess I’ll just sum it up what I submitted and
say, I’m thoroughly enjoying your blog. I as well am an aspiring blog
blogger but I’m still new to the whole thing. Do you have any suggestions for beginner blog writers?
I’d genuinely appreciate it.
August 27, 2023 at 8:36 am
Woah! I’m really digging the template/theme of this website.
It’s simple, yet effective. A lot of times it’s very difficult to get
that “perfect balance” between superb usability and visual appeal.
I must say you’ve done a very good job with this.
Additionally, the blog loads very quick for me on Chrome.
Exceptional Blog!
August 27, 2023 at 12:58 pm
I would love to add when you do not surely have an insurance policy or you do not remain in any group insurance, you might well benefit from seeking aid from a health insurance broker. Self-employed or those with medical conditions typically seek the help of one health insurance specialist. Thanks for your blog post.
August 27, 2023 at 10:59 pm
Hurrah! After all I got a webpage from where I be
able to in fact get helpful information regarding my
study and knowledge.
August 28, 2023 at 5:48 am
cialis first time experiences cialis 20mg prix en pharmacie cialis original 20mg
August 29, 2023 at 2:00 am
Hello there! This is kind of off topic but I need some advice from an established blog. Is it difficult to set up your own blog? I’m not very techincal but I can figure things out pretty quick. I’m thinking about creating my own but I’m not sure where to begin. Do you have any points or suggestions? Many thanks
August 29, 2023 at 9:51 am
Greetings! I know this is kinda off topic nevertheless I’d figured I’d ask.
Would you be interested in trading links or maybe guest authoring a blog
post or vice-versa? My site goes over a lot of the same topics
as yours and I believe we could greatly benefit from each other.
If you’re interested feel free to shoot me an e-mail.
I look forward to hearing from you! Terrific blog by the way!
August 29, 2023 at 11:02 am
Thanks for sharing your ideas. A very important factor is that students have a solution between federal government student loan plus a private student loan where it really is easier to go with student loan debt consolidation loan than over the federal student loan.
August 29, 2023 at 12:57 pm
Pretty! This was an incredibly wonderful article. Thank
you for providing this information.
August 29, 2023 at 1:04 pm
Aw, this was a very nice post. In thought I wish to put in writing like this moreover ? taking time and precise effort to make an excellent article? but what can I say? I procrastinate alot and by no means appear to get something done.
August 30, 2023 at 2:57 am
This is my first time pay a quick visit at here and i am in fact pleassant to read everthing at one place.
August 30, 2023 at 9:02 pm
I enjoy what you guys are usually up too. Such clever work and
reporting! Keep up the great works guys I’ve included you guys to my own blogroll.
August 30, 2023 at 11:09 pm
Do you mind if I quote a few of your articles as long as I provide credit and sources back to your webpage?
My blog is in the exact same area of interest as yours and
my visitors would genuinely benefit from a lot of the information you provide here.
Please let me know if this ok with you. Appreciate it!
August 30, 2023 at 11:55 pm
Excellent beat ! I would like to apprentice while you amend your web site,
how can i subscribe for a blog website? The account helped me a acceptable deal.
I had been a little bit acquainted of this
your broadcast offered bright clear concept
August 31, 2023 at 12:34 am
Keep this going please, great job!
August 31, 2023 at 12:47 am
In accordance with my study, after a in foreclosure process home is bought at an auction, it is common with the borrower in order to still have a remaining balance on the bank loan. There are many loan providers who attempt to have all charges and liens cleared by the upcoming buyer. Nonetheless, depending on particular programs, restrictions, and state guidelines there may be quite a few loans which are not easily resolved through the shift of financial loans. Therefore, the responsibility still remains on the lender that has had his or her property in foreclosure. Thanks for sharing your opinions on this blog site.
August 31, 2023 at 5:24 am
Normally I don’t learn post on blogs, however I wish to say
that this write-up very forced me to take a look at and do so!
Your writing style has been surprised me. Thanks, quite nice post.
August 31, 2023 at 8:00 am
Hi my friend! I wish to say that this article is amazing, nice written and include almost all important infos. I would like to peer more posts like this .
August 31, 2023 at 11:43 am
Wow that was odd. I just wrote an very long comment but after I clicked submit my comment didn’t show up. Grrrr… well I’m not writing all that over again. Anyways, just wanted to say great blog!
September 1, 2023 at 1:25 am
From my investigation, shopping for electronics online may be easily expensive, but there are some tips that you can use to help you get the best things. There are continually ways to discover discount promotions that could help make one to buy the best electronic products products at the lowest prices. Great blog post.
September 2, 2023 at 5:16 am
I am sure this post has touched all the internet people, its really really pleasant paragraph on building up new website.
September 2, 2023 at 4:44 pm
you are in point of fact a just right webmaster. The site loading speed is incredible. It sort of feels that you are doing any unique trick. Furthermore, The contents are masterwork. you have performed a magnificent job on this topic!
September 2, 2023 at 8:22 pm
Hiya! Quick question that’s totally off topic. Do you know how to make your site mobile friendly?
My website looks weird when viewing from my apple iphone.
I’m trying to find a template or plugin that might be able to fix this
problem. If you have any suggestions, please
share. Many thanks!
September 2, 2023 at 10:16 pm
With havin so much written content do you ever run into any issues of plagorism or copyright infringement?
My website has a lot of unique content I’ve either created myself or outsourced but it seems a
lot of it is popping it up all over the internet without my
permission. Do you know any methods to help reduce content from being stolen?
I’d certainly appreciate it.
September 3, 2023 at 2:17 am
Wonderful beat ! I wish to apprentice while you amend your web site,
how could i subscribe for a blog web site? The account aided
me a acceptable deal. I had been a little bit acquainted of this your broadcast provided bright clear idea
September 3, 2023 at 7:27 am
Hey there, I think your blog might be having browser compatibility issues.
When I look at your blog in Safari, it looks fine but when opening
in Internet Explorer, it has some overlapping. I just wanted to give you
a quick heads up! Other then that, superb blog!
September 3, 2023 at 9:03 am
This really answered my drawback, thanks!
September 3, 2023 at 3:58 pm
Please let me know if you’re looking for a article writer for your site.
You have some really good posts and I think I would be a good asset.
If you ever want to take some of the load off, I’d absolutely love
to write some content for your blog in exchange for a link back to mine.
Please blast me an email if interested. Thank you!
September 3, 2023 at 8:52 pm
Greetings from Idaho! I’m bored to death at work so I decided to check out
your website on my iphone during lunch break. I love the knowledge you present here and can’t wait
to take a look when I get home. I’m shocked at how quick your blog loaded on my mobile ..
I’m not even using WIFI, just 3G .. Anyhow, great blog!
September 4, 2023 at 1:38 am
When I initially commented I clicked the -Notify me when new comments are added- checkbox and now every time a comment is added I get 4 emails with the identical comment. Is there any means you possibly can remove me from that service? Thanks!
September 4, 2023 at 11:26 am
My family members every time say that I am killing my time here at net, but I know I am getting knowledge all the time
by reading thes pleasant articles.
September 4, 2023 at 5:12 pm
I would like to thnkx for the efforts you have put in writing this web site. I’m hoping the same high-grade blog post from you in the upcoming also. In fact your creative writing skills has encouraged me to get my own website now. Actually the blogging is spreading its wings rapidly. Your write up is a good example of it.
September 4, 2023 at 10:17 pm
My brother recommended I might like this website. He was totally right. This post actually made my day. You can not imagine just how much time I had spent for this info! Thanks!
September 5, 2023 at 6:02 pm
Spot on with this write-up, I really think this website needs rather more consideration. I?ll in all probability be again to learn rather more, thanks for that info.
September 6, 2023 at 3:09 am
Do you have a spam problem on this website; I also am a blogger, and
I was curious about your situation; many of us have created some nice methods and
we are looking to trade solutions with others,
why not shoot me an email if interested.
September 6, 2023 at 3:56 am
Hi, I want to subscribe for this webpage to obtain newest updates,
so where can i do it please help out.
September 6, 2023 at 4:59 am
Whats up are using WordPress for your site platform? I’m new to
the blog world but I’m trying to get started and set up my own. Do
you need any coding knowledge to make your own blog?
Any help would be greatly appreciated!
September 6, 2023 at 12:01 pm
I need to to thank you for this excellent read!! I
certainly loved every bit of it. I have you book marked to look at new things you post…
September 6, 2023 at 12:59 pm
Hello everyone, it’s my first visit at this website, and paragraph is genuinely fruitful
in favor of me, keep up posting these types of articles.
September 6, 2023 at 9:44 pm
Hey There. I found your blog using msn. This is an extremely well written article.
I’ll be sure to bookmark it and return to read more of your useful information. Thanks for the post.
I will certainly return.
September 7, 2023 at 12:57 am
whoah this blog is wonderful i love studying your articles.
Stay up the great work! You recognize, a lot of persons are hunting round for this information, you can help them greatly.
September 7, 2023 at 5:11 am
It’s an amazing piece of writing designed for all the web visitors; they will
take benefit from it I am sure.
September 7, 2023 at 6:10 am
Howdy! This is my first comment here so I just wanted to give a
quick shout out and say I genuinely enjoy reading through your posts.
Can you recommend any other blogs/websites/forums that cover the same subjects?
Thanks!
September 7, 2023 at 10:13 am
Hello there, I discovered your website by means of Google whilst searching for a
similar topic, your site got here up, it
appears to be like great. I’ve bookmarked it in my google bookmarks.
Hi there, just became aware of your weblog through Google,
and found that it is really informative. I am gonna be careful for brussels.
I’ll be grateful for those who proceed this in future.
Lots of people can be benefited out of your writing.
Cheers!
December 20, 2023 at 5:42 am
I am inexorably drawn to the mesmerizing tapestry of your words, interwoven with polysyllabic expressions and labyrinthine syntax, rendering each blog post an intellectual marvel that leaves me in awe.