Phase I Trials
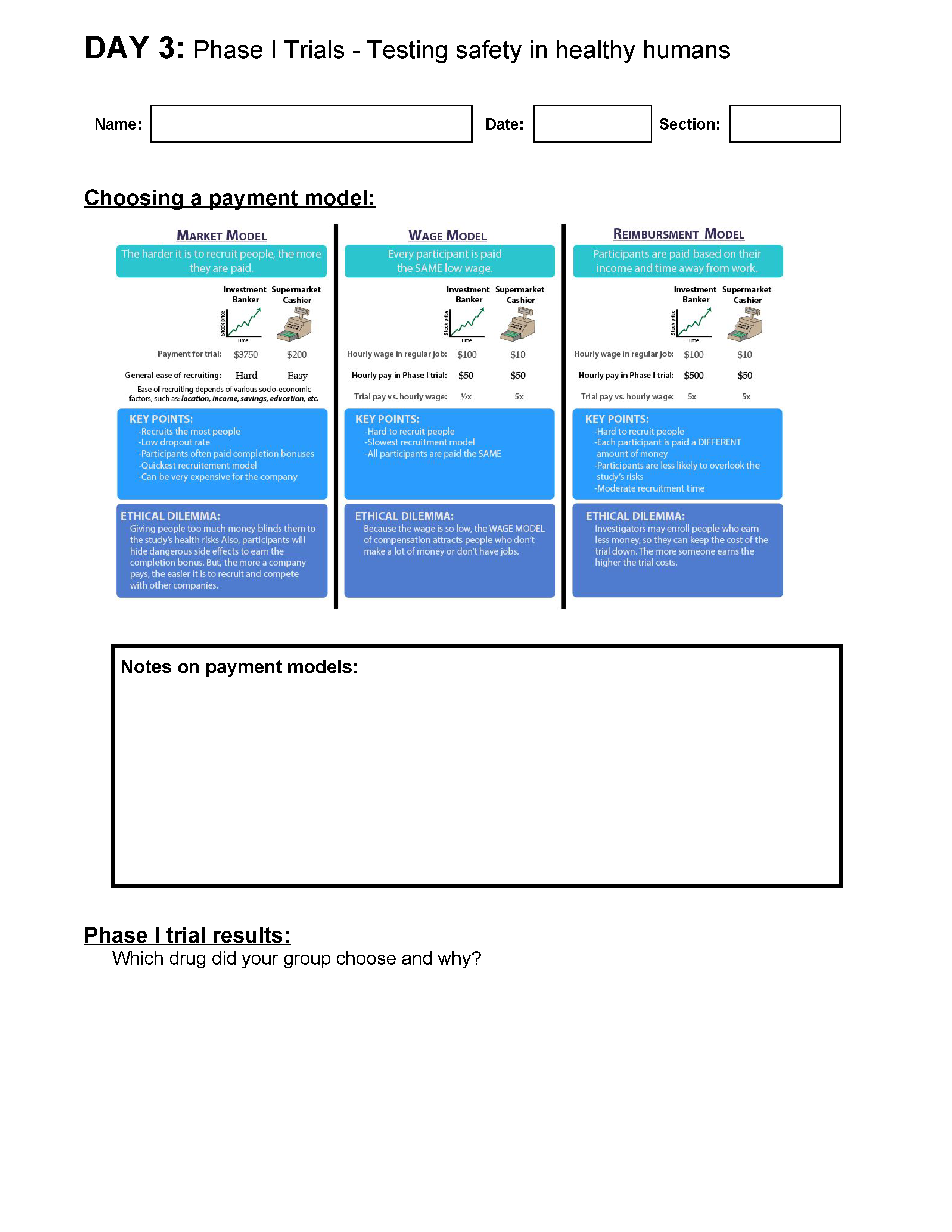
After testing a drug in an animal model (like mice), a drug is moved to clinical trials, where it is tested on people. During Phase I clinical trials the drug is tested in a small group of healthy individuals to determine how toxic the drug is (how much damage a drug does to the body). This is the stage at which dosing is determined. In today’s lesson, students will discuss how the company will pay their Phase I trial participants (there are three main models), and then analyze the toxicity and side effect data from testing three of the drugs in healthy humans. Students will choose one drug to send to Phase II/III trials.
In the tabs below, you’ll find the information you need to prepare for class. To access copies of the slides, workbook, and teacher resources, click the Box download (for .pptx, .docx, and PDF files) or the link to the Google Drive folder at the bottom of the page.
Overview
Learning Objectives:
- Students will be able to compare and contrast different payment models for volunteers of Phase I clinical trials, and recommend one that the company should use.
- Students will be able to state the purpose of Phase I Trials.
- Students will analyze data from Phase I Trials and decide as a group which drug to move forward to Phase II/III trials: Etravirine, Raltegravir, or Pro 542.
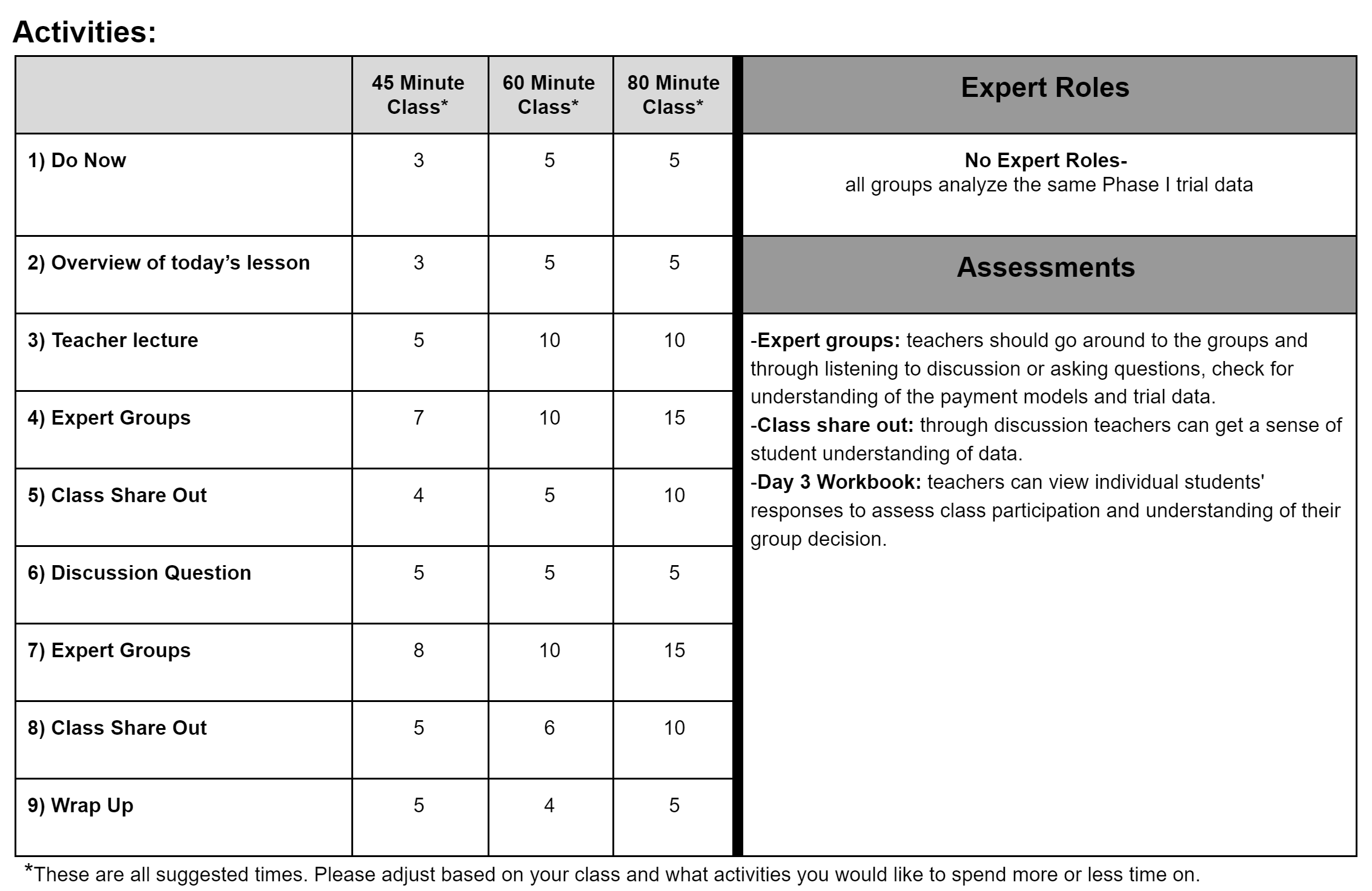
Activities:
Daily Slides
Workbook