Phase II/III Trials
In today’s lesson, the class will be choosing one drug to submit to the FDA (Food and Drug Administration) for approval. This decision will be based on various perspectives and data that will help the class determine whether it is financially risky to continue with the chosen drug and whether the benefits outweigh the risks for HIV patients. Some of the information students will analyze is data from Phase II/III trials, in which the drug is tested in HIV patients for both toxicity and efficacy at reducing HIV viral loads. Other information students will analyze includes drug resistance data, projected revenue, and factors that affect the drug’s marketability such as efficacy, cost, and the particular viral targets of the drugs.
In the tabs below, you’ll find the information you need to prepare for class. To access copies of the slides, workbook, and teacher resources, click the Box download (for .pptx, .docx, and PDF files) or the link to the Google Drive folder at the bottom of the page.
Overview
Learning Objectives:
- Students will analyze data from different career roles and decide as a group which drug to put forward for FDA approval: Etravirine or Pro 542.
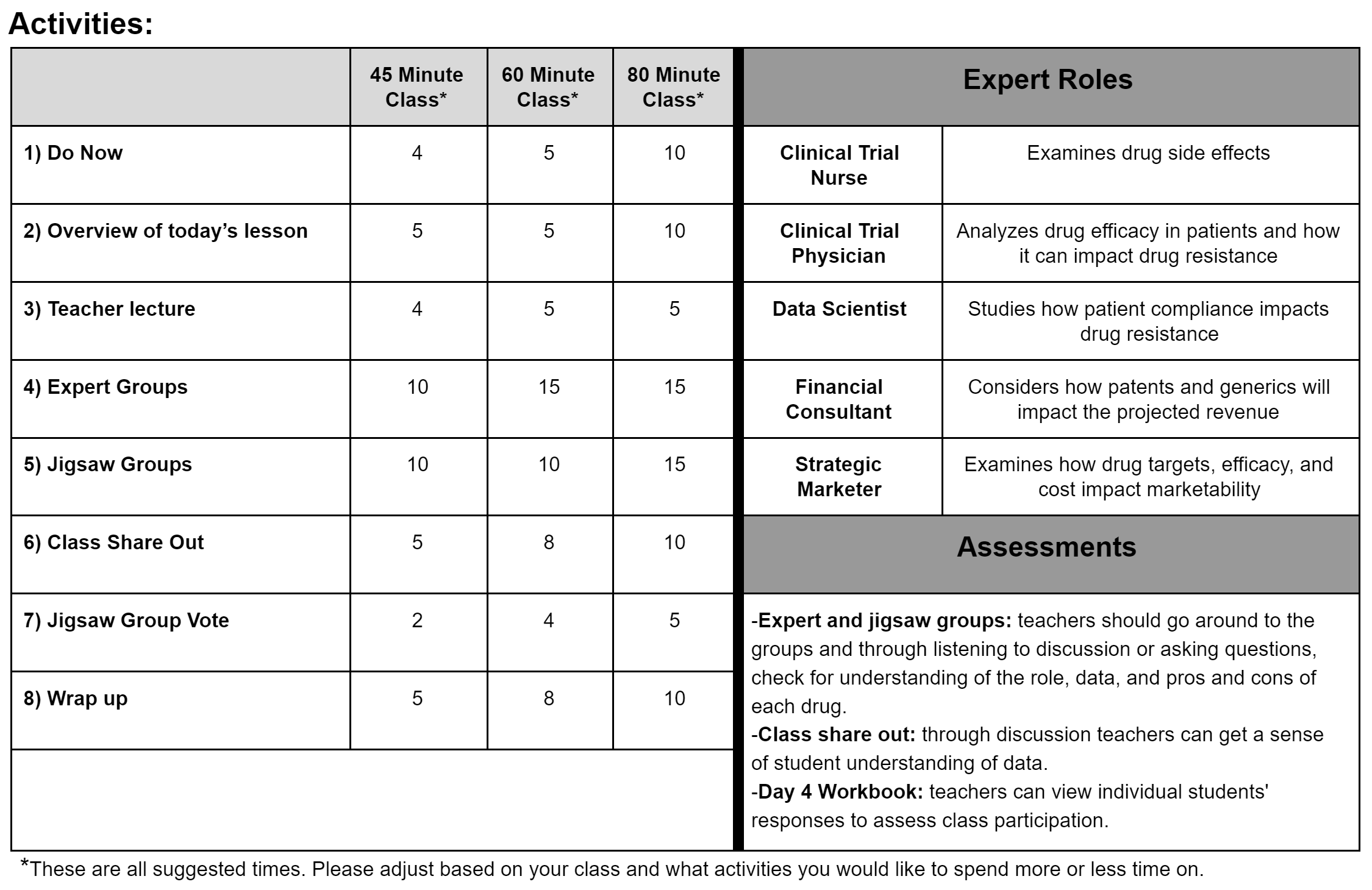
Activities:
Daily Slides
Workbook





